Abstract
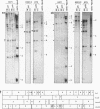
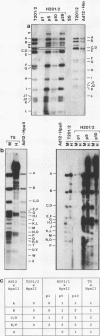
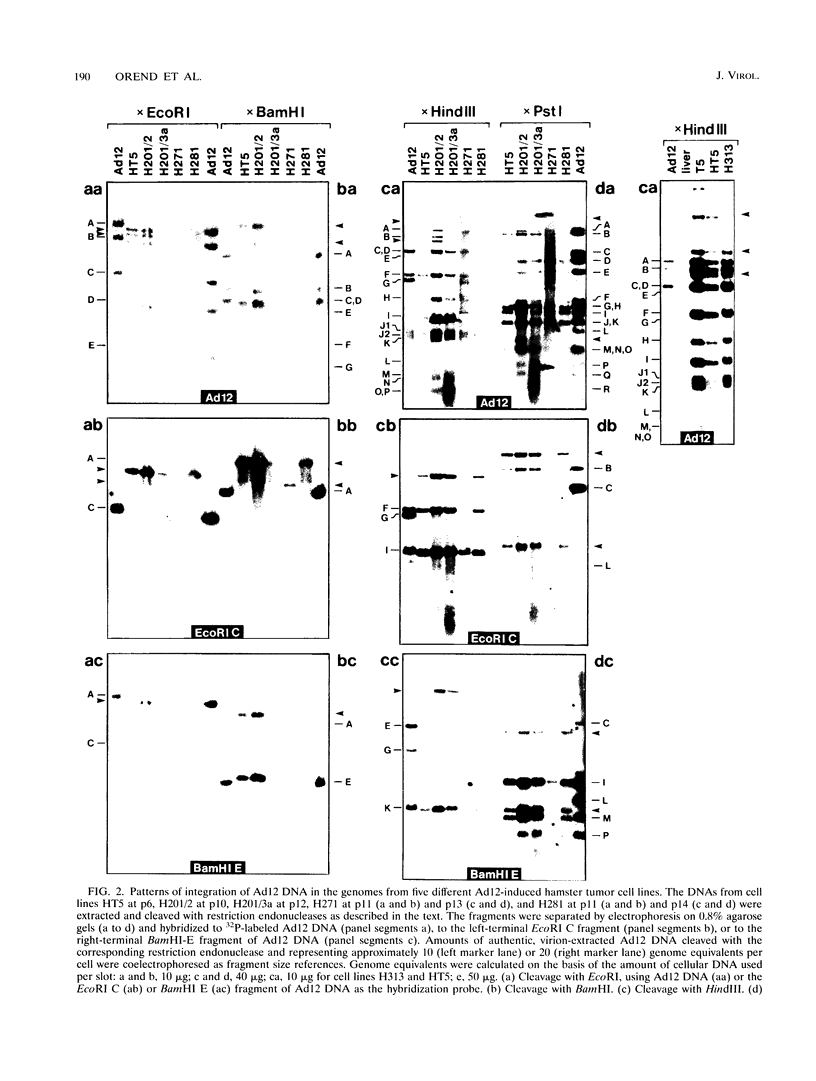
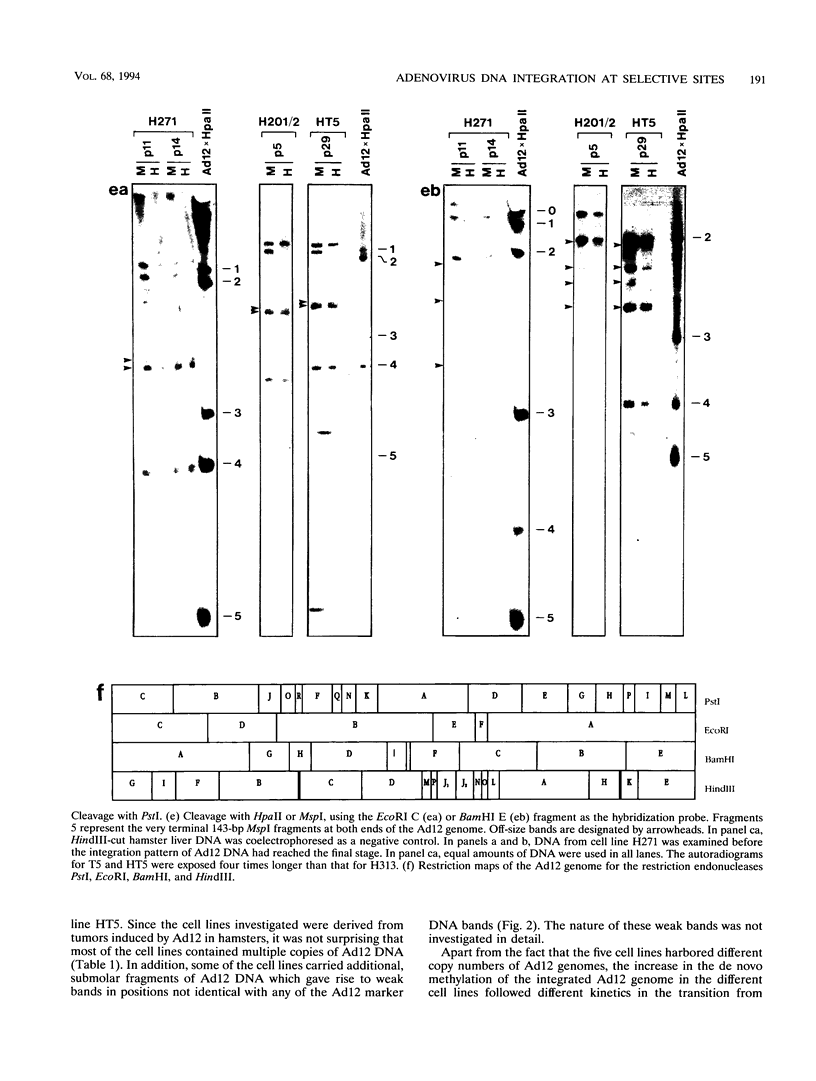
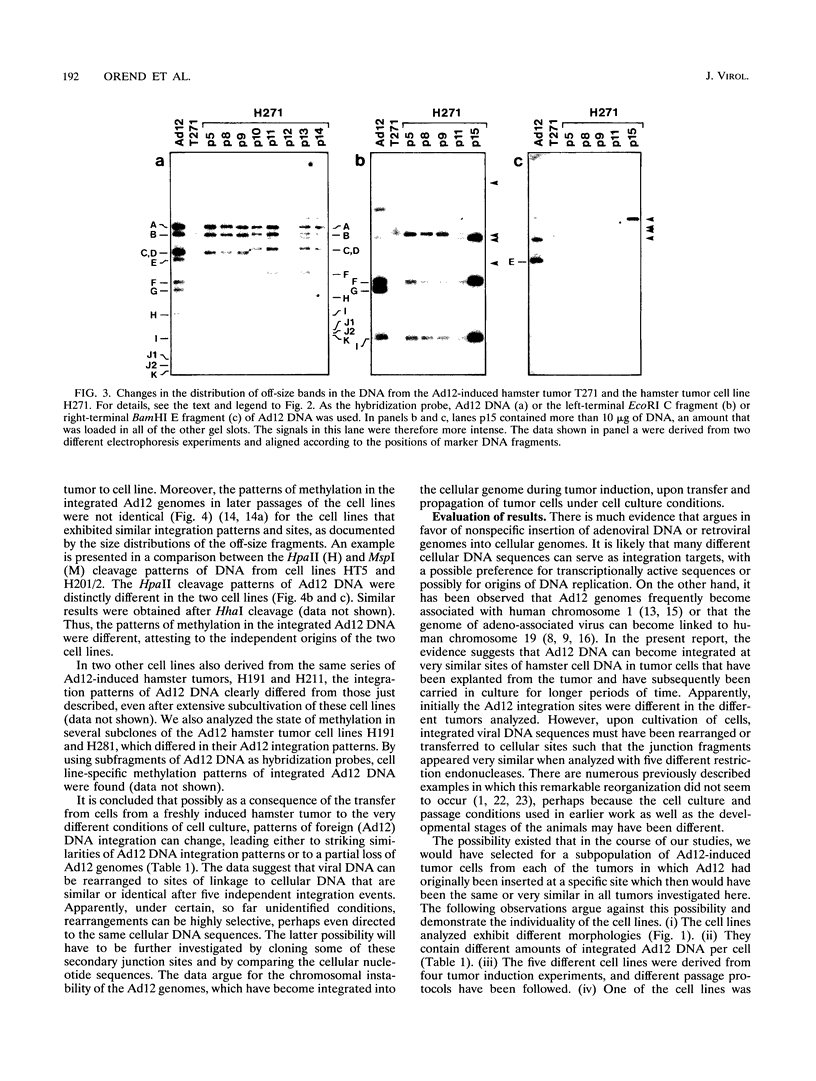
We investigated whether, upon the integration of multiple copies of adenovirus type 12 (Ad12) DNA into an established mammalian (hamster) genome, the pattern of foreign DNA insertion would remain stable or change with consecutive passages of cells in culture. By the injection of purified Ad12 into newborn hamsters, tumors were induced, cells from these tumors were cultivated, and five independent cell lines, HT5, H201/2, H201/3, H271, and H281, were established. These cell lines carried different copy numbers of Ad12 DNA per cell in an integrated form and differed in morphology. Cell line HT5 had been passed twice through hamsters as tumor cells and was subsequently passaged in culture. Patterns of Ad12 DNA integration were determined by restriction cleavage of the nuclear DNA with BamHI, EcoRI, HindIII, MspI, or PstI followed by Southern blot hybridization using 32P-labeled Ad12 DNA or its cloned terminal DNA fragments as hybridization probes. In this way, the off-size fragments, which represented the sites of linkage between Ad12 and cellular DNAs, were determined. At early passage levels in culture, the integration sites of Ad12 DNA in the hamster genome, as characterized by the positions of off-size fragments in agarose or polyacrylamide gel electrophoresis, were different in the five different tumor cell lines. Upon repeated passage, however, the off-size fragment patterns generated by the five restriction endonucleases became very similar in the five tumor cell lines. This surprising result indicates that under cell culture conditions, Ad12-transformed tumor cell lines that carry the foreign (Ad12) genome in selective, probably very similar sites of the cellular genome evolve.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doerfler W., Gahlmann R., Stabel S., Deuring R., Lichtenberg U., Schulz M., Eick D., Leisten R. On the mechanism of recombination between adenoviral and cellular DNAs: the structure of junction sites. Curr Top Microbiol Immunol. 1984;109:193–228. doi: 10.1007/978-3-642-69460-8_9. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Uptake, fixation, and expression of foreign DNA in mammalian cells: the organization of integrated adenovirus DNA sequences. Curr Top Microbiol Immunol. 1982;101:127–194. doi: 10.1007/978-3-642-68654-2_6. [DOI] [PubMed] [Google Scholar]
- Groneberg J., Sutter D., Soboll H., Doerfler W. Morphological revertants of adenovirus type 12-transformed hamster cells. J Gen Virol. 1978 Sep;40(3):635–645. doi: 10.1099/0022-1317-40-3-635. [DOI] [PubMed] [Google Scholar]
- Jessberger R., Heuss D., Doerfler W. Recombination in hamster cell nuclear extracts between adenovirus type 12 DNA and two hamster preinsertion sequences. EMBO J. 1989 Mar;8(3):869–878. doi: 10.1002/j.1460-2075.1989.tb03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger R., Weisshaar B., Stabel S., Doerfler W. Arrangement and expression of integrated adenovirus type 12 DNA in the transformed hamster cell line HA12/7: amplification of Ad12 and c-myc DNAs and evidence for hybrid viral-cellular transcripts. Virus Res. 1989 Jun;13(2):113–128. doi: 10.1016/0168-1702(89)90010-5. [DOI] [PubMed] [Google Scholar]
- Koetsier P. A., Schorr J., Doerfler W. A rapid optimized protocol for downward alkaline Southern blotting of DNA. Biotechniques. 1993 Aug;15(2):260–262. [PubMed] [Google Scholar]
- Kotin R. M., Linden R. M., Berns K. I. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992 Dec;11(13):5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotin R. M., Menninger J. C., Ward D. C., Berns K. I. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991 Jul;10(3):831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- Kuhlmann I., Achten S., Rudolph R., Doerfler W. Tumor induction by human adenovirus type 12 in hamsters: loss of the viral genome from adenovirus type 12-induced tumor cells is compatible with tumor formation. EMBO J. 1982;1(1):79–86. doi: 10.1002/j.1460-2075.1982.tb01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann I., Doerfler W. Shifts in the extent and patterns of DNA methylation upon explanation and subcultivation of adenovirus type 12-induced hamster tumor cells. Virology. 1982 Apr 15;118(1):169–180. doi: 10.1016/0042-6822(82)90330-0. [DOI] [PubMed] [Google Scholar]
- Lichtenberg U., Zock C., Doerfler W. Insertion of adenovirus type 12 DNA in the vicinity of an intracisternal A particle genome in Syrian hamster tumor cells. J Virol. 1987 Sep;61(9):2719–2726. doi: 10.1128/jvi.61.9.2719-2726.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall J. K., Dunn A. R., Jones K. W. In situ hybridization of adenovirus RNA and DNA. Nature. 1972 Apr 14;236(5346):346–348. doi: 10.1038/236346a0. [DOI] [PubMed] [Google Scholar]
- Orend G., Kuhlmann I., Doerfler W. Spreading of DNA methylation across integrated foreign (adenovirus type 12) genomes in mammalian cells. J Virol. 1991 Aug;65(8):4301–4308. doi: 10.1128/jvi.65.8.4301-4308.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosahl T., Doerfler W. Predominant association of adenovirus type 12 DNA with human chromosome 1 early in productive infection. Virology. 1988 Feb;162(2):494–497. doi: 10.1016/0042-6822(88)90494-1. [DOI] [PubMed] [Google Scholar]
- Samulski R. J., Zhu X., Xiao X., Brook J. D., Housman D. E., Epstein N., Hunter L. A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991 Dec;10(12):3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr J., Doerfler W. Non-homologous recombination between adenovirus and AcNPV DNA fragments in cell-free extracts from insect Spodoptera frugiperda nuclei. Virus Res. 1993 May;28(2):153–170. doi: 10.1016/0168-1702(93)90133-8. [DOI] [PubMed] [Google Scholar]
- Schulz M., Freisem-Rabien U., Jessberger R., Doerfler W. Transcriptional activities of mammalian genomes at sites of recombination with foreign DNA. J Virol. 1987 Feb;61(2):344–353. doi: 10.1128/jvi.61.2.344-353.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sprengel J., Schmitz B., Heuss-Neitzel D., Zock C., Doerfler W. Nucleotide sequence of human adenovirus type 12 DNA: comparative functional analysis. J Virol. 1994 Jan;68(1):379–389. doi: 10.1128/jvi.68.1.379-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Doerfler W., Friis R. R. Integration sites of adenovirus type 12 DNA in transformed hamster cells and hamster tumor cells. J Virol. 1980 Oct;36(1):22–40. doi: 10.1128/jvi.36.1.22-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Doerfler W. Nucleotide sequence at the site of junction between adenovirus type 12 DNA and repetitive hamster cell DNA in transformed cell line CLAC1. Nucleic Acids Res. 1982 Dec 20;10(24):8007–8023. doi: 10.1093/nar/10.24.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978 Jul;14(3):569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- Tatzelt J., Fechteler K., Langenbach P., Doerfler W. Fractionated nuclear extracts from hamster cells catalyze cell-free recombination at selective sequences between adenovirus DNA and a hamster preinsertion site. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7356–7360. doi: 10.1073/pnas.90.15.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatzelt J., Scholz B., Fechteler K., Jessberger R., Doerfler W. Recombination between adenovirus type 12 DNA and a hamster preinsertion sequence in a cell-free system. Patch homologies and fractionation of nuclear extracts. J Mol Biol. 1992 Jul 5;226(1):117–126. doi: 10.1016/0022-2836(92)90128-7. [DOI] [PubMed] [Google Scholar]