Abstract
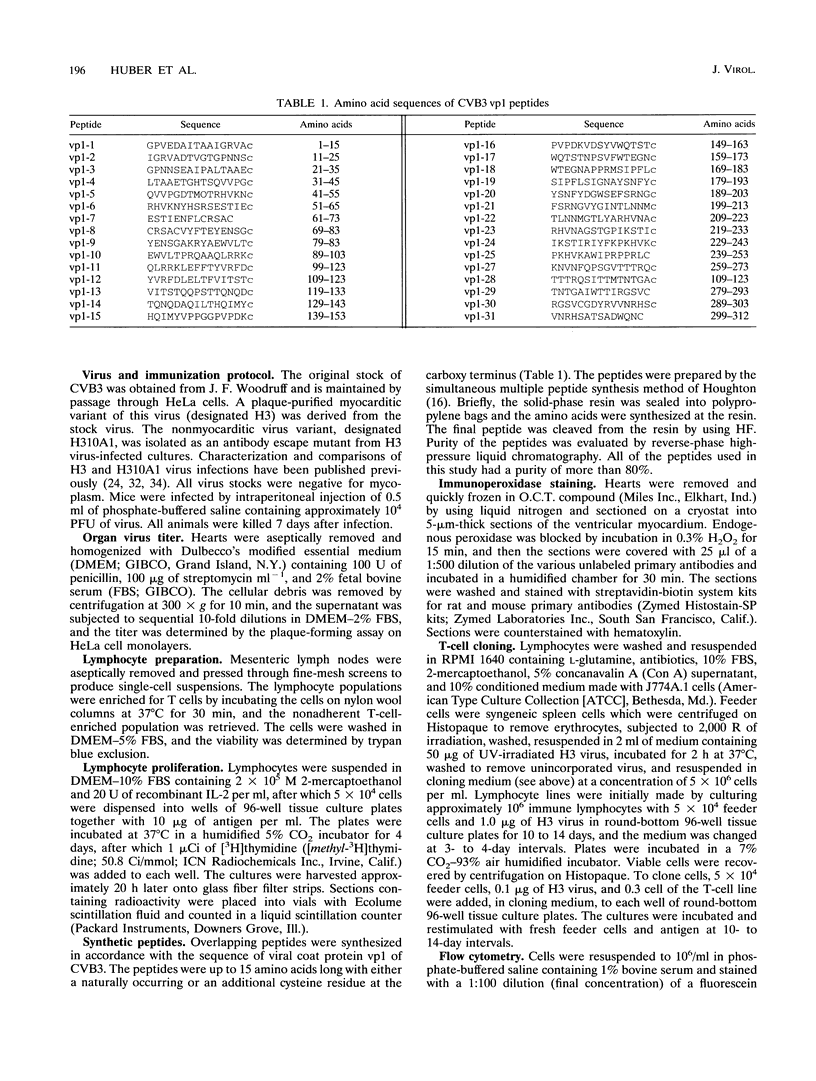
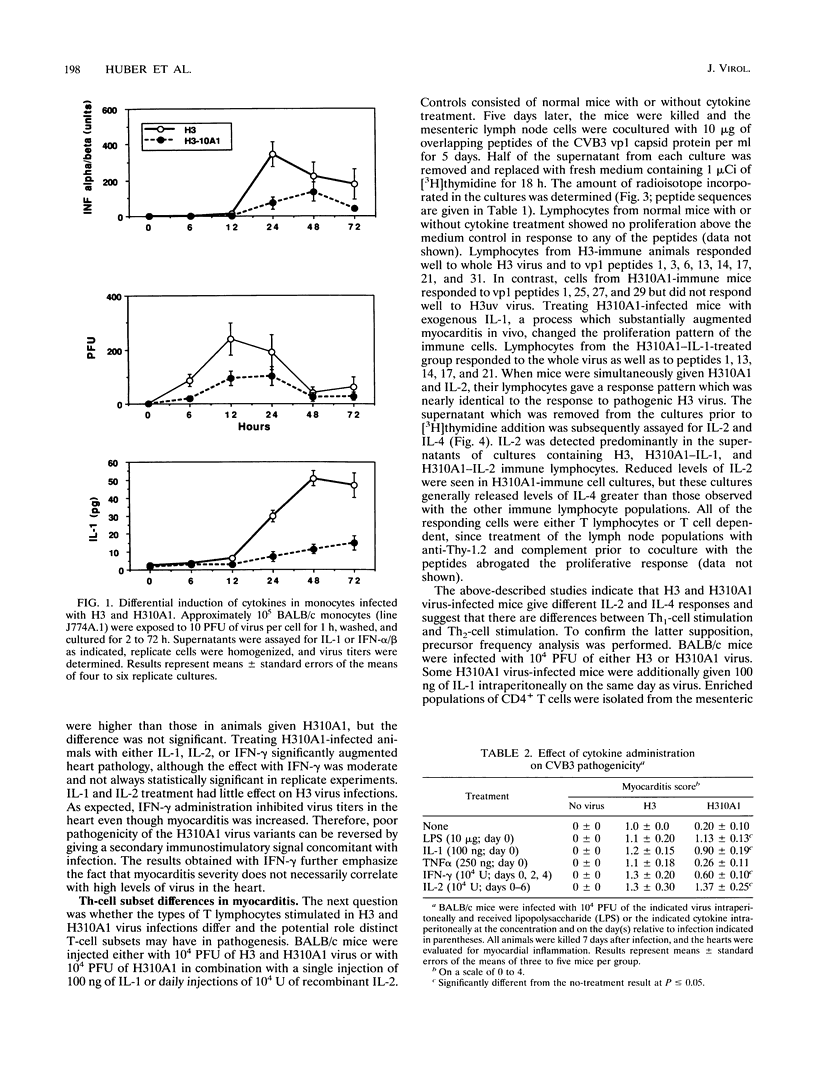
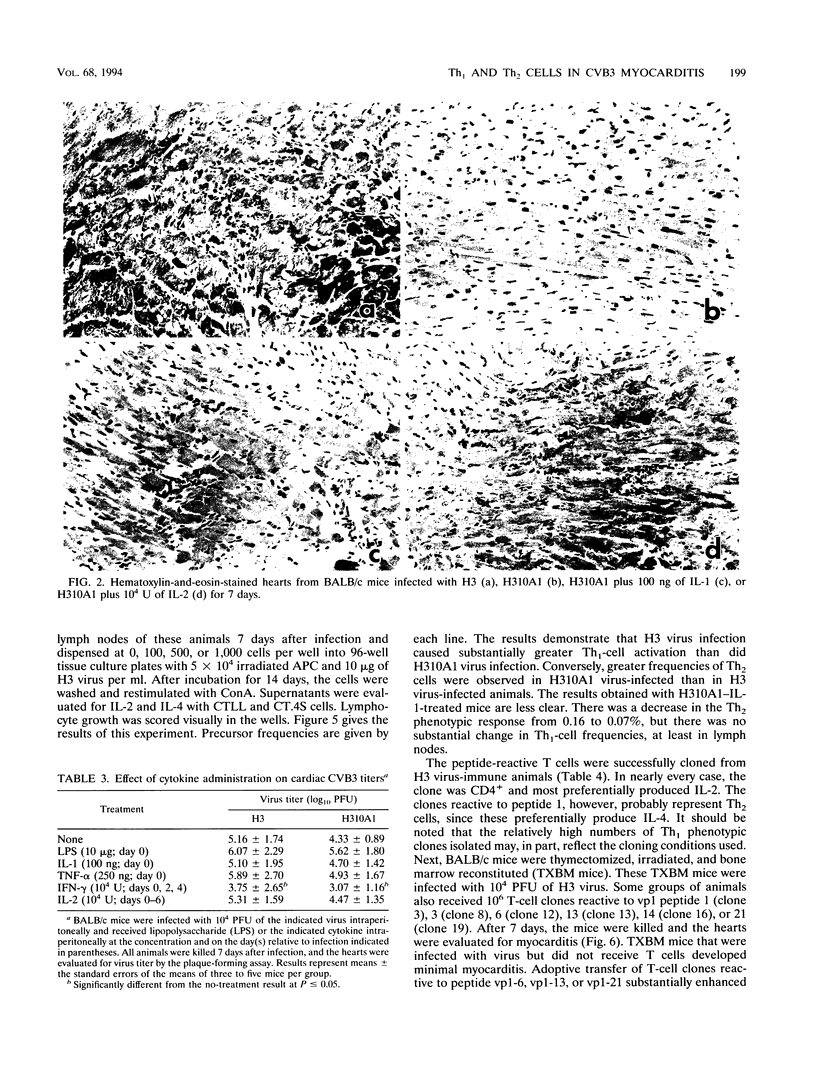
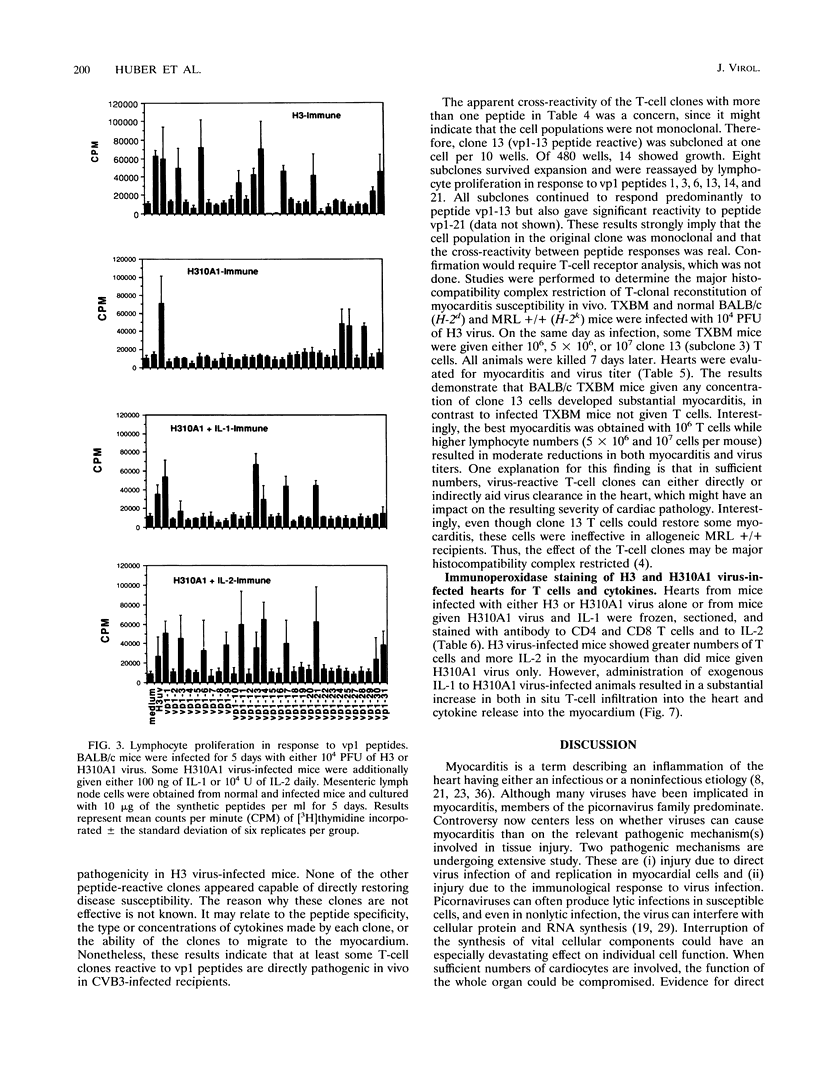
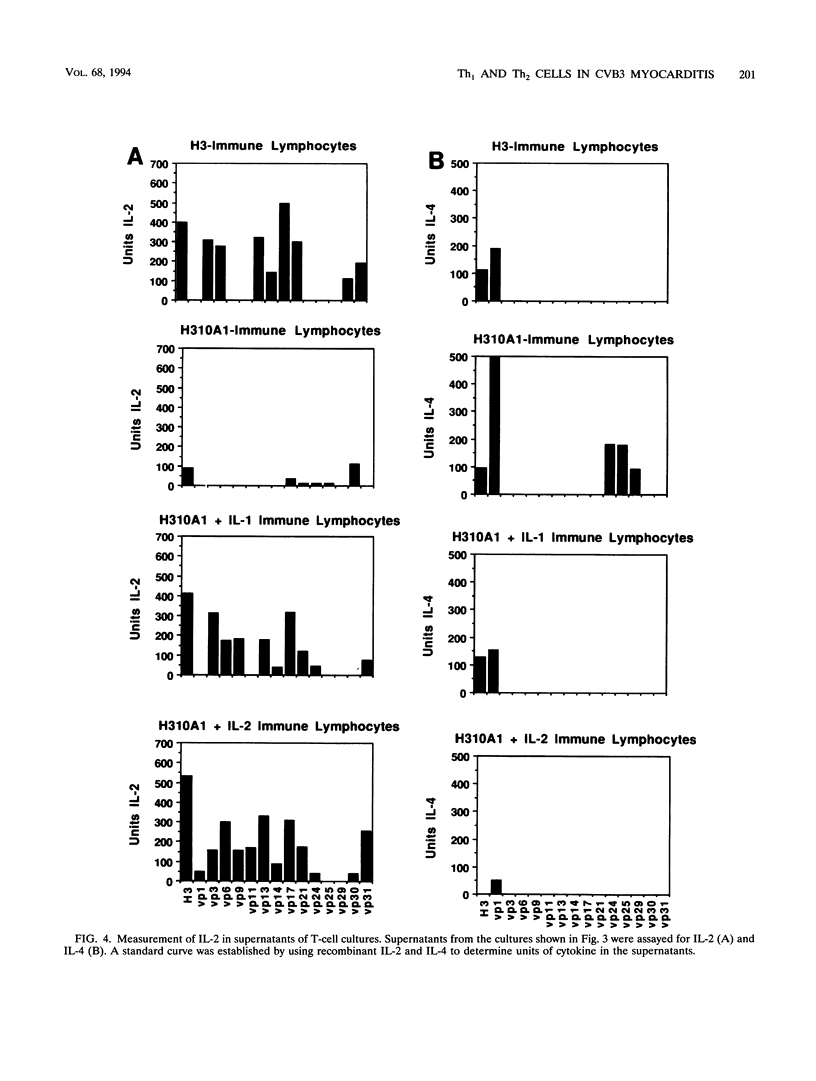
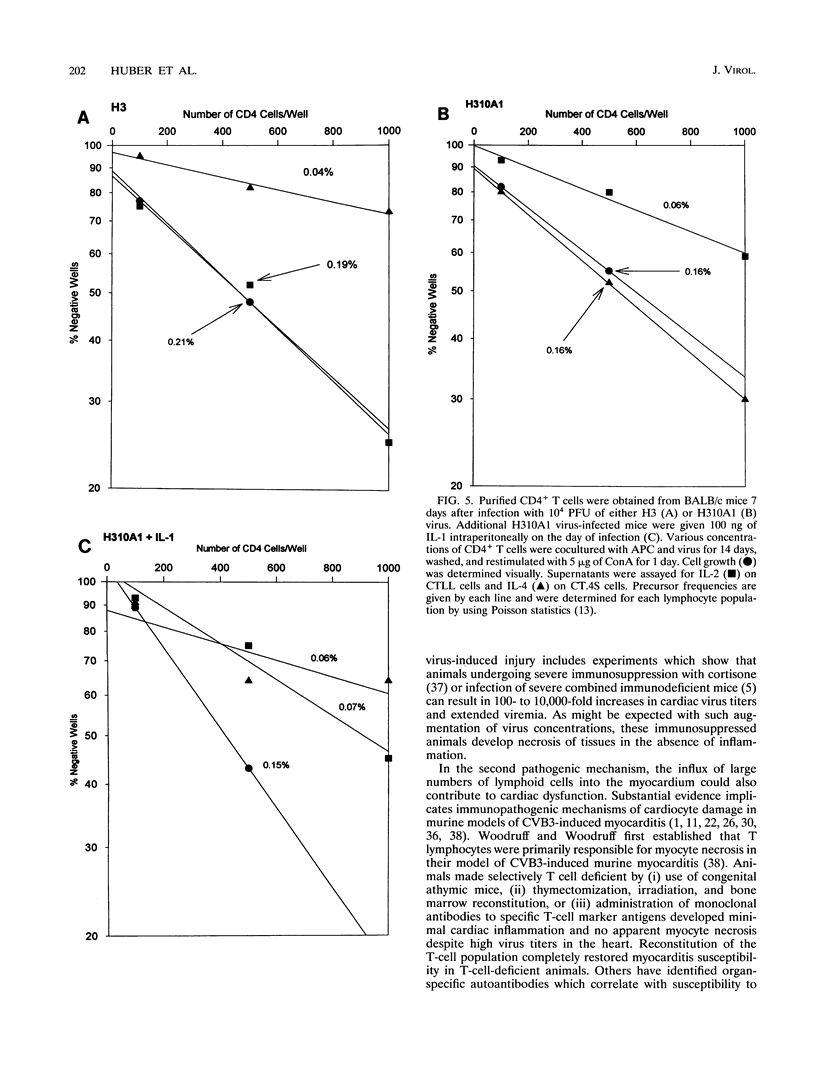
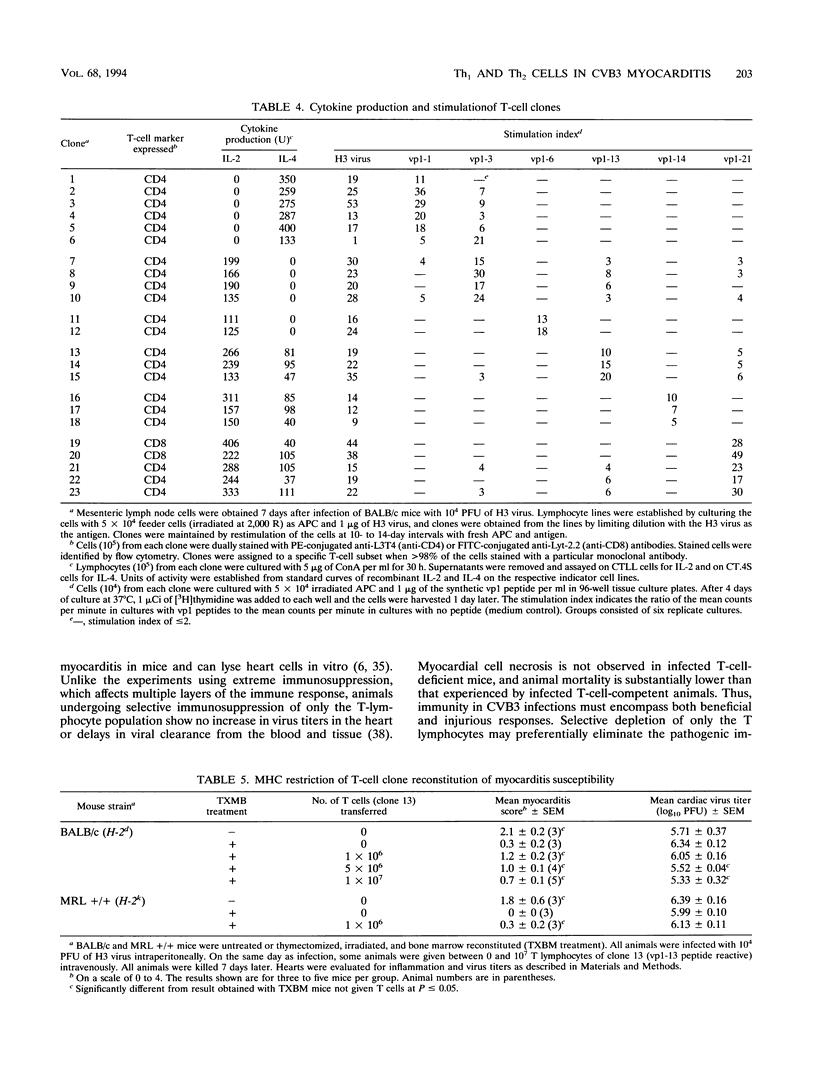
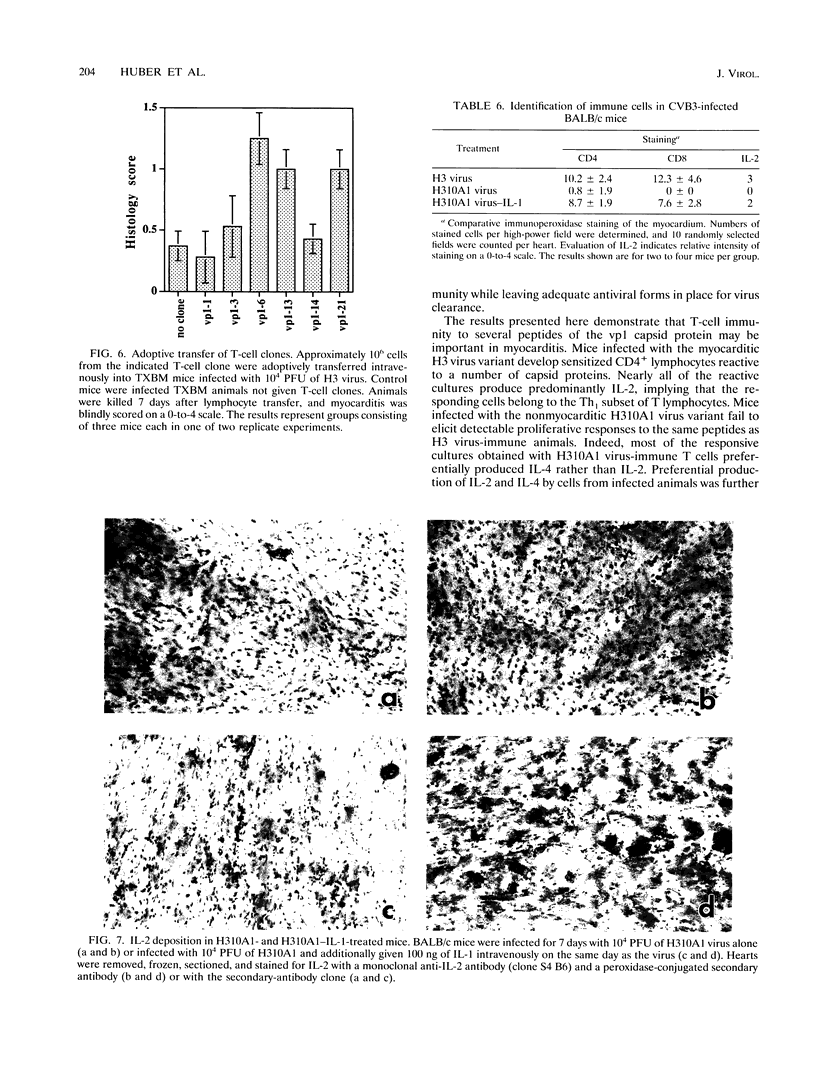
Two variants of coxsackievirus B3 (CVB3) which differ dramatically in the ability to induce myocarditis in BALB/c mice were studied. H3 virus infection of murine monocytes in vitro resulted in release of concentrations of interleukin 1 (IL-1) and alpha/beta interferon that were high compared with those of cells infected with the H310A1 virus variant. In vivo, H3 virus infection caused substantial inflammatory cell infiltration of the myocardium, and lymphocytes from these animals gave predominantly Th1-cell responses to either whole H3 virus or overlapping peptides of the CVB3 vp1 capsid protein, as determined by IL-2 production. In contrast, H310A1 virus infection produced minimal myocarditis and Th1-cell responses, but Th2-cell activation was more pronounced than in H3 virus-infected mice (as determined by IL-4 concentrations). Exogenous treatment of H310A1 virus-infected mice with either IL-1 or IL-2 restored both myocarditis susceptibility and Th1-cell responses to whole virus and vp1 peptides. Furthermore, H310A1 virus-infected mice given exogenous IL-1 showed substantial in situ IL-2 deposition in the myocardium. These results indicate that CVB3-induced myocarditis may depend upon release of specific cytokines during infection and that activation of Th1 cells may be an important factor in pathogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisel K. W., Srinivasappa J., Prabhakar B. S. Molecular cloning of a heart antigen that cross-reacts with a neutralizing antibody to Coxsackievirus B4. Eur Heart J. 1991 Aug;12 (Suppl 500):60–64. doi: 10.1093/eurheartj/12.suppl_d.60. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Salgame P., Diamond B. Revisiting and revising suppressor T cells. Immunol Today. 1992 Apr;13(4):131–136. doi: 10.1016/0167-5699(92)90110-S. [DOI] [PubMed] [Google Scholar]
- Bogen S. A., Fogelman I., Abbas A. K. Analysis of IL-2, IL-4, and IFN-gamma-producing cells in situ during immune responses to protein antigens. J Immunol. 1993 May 15;150(10):4197–4205. [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. H., Beisel K. W., McManus B. M. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab Invest. 1992 Jan;66(1):24–31. [PubMed] [Google Scholar]
- Cunningham M. W., Antone S. M., Gulizia J. M., McManus B. M., Fischetti V. A., Gauntt C. J. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrin M., Smith C., Huber S. Antigen-specific suppressor T cells prevent cardiac injury in Balb/c mice infected with a nonmyocarditic variant of coxsackievirus group B, type 3. Am J Pathol. 1986 Dec;125(3):578–584. [PMC free article] [PubMed] [Google Scholar]
- Fenoglio J. J., Jr, Ursell P. C., Kellogg C. F., Drusin R. E., Weiss M. B. Diagnosis and classification of myocarditis by endomyocardial biopsy. N Engl J Med. 1983 Jan 6;308(1):12–18. doi: 10.1056/NEJM198301063080103. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Gauntt C. J., Arizpe H. M., Higdon A. L., Rozek M. M., Crawley R., Cunningham M. W. Anti-Coxsackievirus B3 neutralizing antibodies with pathological potential. Eur Heart J. 1991 Aug;12 (Suppl 500):124–129. doi: 10.1093/eurheartj/12.suppl_d.124. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Trousdale M. D., LaBadie D. R., Paque R. E., Nealon T. Properties of coxsackievirus B3 variants which are amyocarditic or myocarditic for mice. J Med Virol. 1979;3(3):207–220. doi: 10.1002/jmv.1890030307. [DOI] [PubMed] [Google Scholar]
- Good M. F., Boyd A. W., Nossal G. J. Analysis of true anti-hapten cytotoxic clones in limit dilution microcultures after correction for "anti-self" activity: precursor frequencies, Ly-2 and Thy-1 phenotype, specificity, and statistical methods. J Immunol. 1983 May;130(5):2046–2055. [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke A., Mohr C., Sprenger H., Graebner C., Stelzner A., Nain M., Gemsa D. Coxsackievirus B3-induced production of tumor necrosis factor-alpha, IL-1 beta, and IL-6 in human monocytes. J Immunol. 1992 Apr 1;148(7):2270–2277. [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Haisch C., Lodge P. A. Functional diversity in vascular endothelial cells: role in coxsackievirus tropism. J Virol. 1990 Sep;64(9):4516–4522. doi: 10.1128/jvi.64.9.4516-4522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Moraska A., Choate M. T cells expressing the gamma delta T-cell receptor potentiate coxsackievirus B3-induced myocarditis. J Virol. 1992 Nov;66(11):6541–6546. doi: 10.1128/jvi.66.11.6541-6546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen G., Detjen B. M., Thach R. E. Shutoff of HeLa cell protein synthesis by encephalomyocarditis virus and poliovirus: a comparative study. J Virol. 1980 Jul;35(1):150–156. doi: 10.1128/jvi.35.1.150-156.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A. M., Wilson F. M. Virus myocardiopathy. Prog Med Virol. 1973;15:63–91. [PubMed] [Google Scholar]
- Lerner A., Wilson F. M., Reyes M. P. Enteroviruses and the heart (with special emphasis on the probable role of coxsackieviruses, group B, types 1-5). II. Observations in humans. Mod Concepts Cardiovasc Dis. 1975 Mar;44(3):11–15. [PubMed] [Google Scholar]
- Leslie K., Blay R., Haisch C., Lodge A., Weller A., Huber S. Clinical and experimental aspects of viral myocarditis. Clin Microbiol Rev. 1989 Apr;2(2):191–203. doi: 10.1128/cmr.2.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon R. P., Moraska A. F., Huber S. A., Schwimmbeck P., Schultheiss P. An attenuated variant of Coxsackievirus B3 preferentially induces immunoregulatory T cells in vivo. J Virol. 1991 Nov;65(11):5813–5819. doi: 10.1128/jvi.65.11.5813-5819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L., Troutt A. B., Handman E., Kelso A. Changes in the precursor frequencies of IL-4 and IFN-gamma secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol. 1992 Oct 15;149(8):2715–2721. [PubMed] [Google Scholar]
- Neu N., Beisel K. W., Traystman M. D., Rose N. R., Craig S. W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987 Apr 15;138(8):2488–2492. [PubMed] [Google Scholar]
- Oswald I. P., Gazzinelli R. T., Sher A., James S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992 Jun 1;148(11):3578–3582. [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmbeck P. L., Bland N. K., Schultheiss H. P., Strauer B. E. The possible value of synthetic peptides in the diagnosis and therapy of myocarditis and dilated cardiomyopathy. Eur Heart J. 1991 Aug;12 (Suppl 500):76–80. doi: 10.1093/eurheartj/12.suppl_d.76. [DOI] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Van Houten N., Bouchard P. E., Moraska A., Huber S. A. Selection of an attenuated Coxsackievirus B3 variant, using a monoclonal antibody reactive to myocyte antigen. J Virol. 1991 Mar;65(3):1286–1290. doi: 10.1128/jvi.65.3.1286-1290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. T., Hawrylowicz C. M., Unanue E. R. T helper cell subsets require the expression of distinct costimulatory signals by antigen-presenting cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8181–8185. doi: 10.1073/pnas.85.21.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A. H., Simpson K., Herzum M., Van Houten N., Huber S. A. Coxsackievirus-B3-induced myocarditis: virus receptor antibodies modulate myocarditis. J Immunol. 1989 Sep 15;143(6):1843–1850. [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Herskowitz A., Rose N. R. Variations in the susceptibility to Coxsackievirus B3-induced myocarditis among different strains of mice. J Immunol. 1986 Mar 1;136(5):1846–1852. [PubMed] [Google Scholar]
- Woodruff J. F. Lack of correlation between neutralizing antibody production and suppression of coxsackievirus B-3 replication in target organs: evidence for involvement of mononuclear inflammatory cells in host defense. J Immunol. 1979 Jul;123(1):31–36. [PubMed] [Google Scholar]
- Woodruff J. F. Viral myocarditis. A review. Am J Pathol. 1980 Nov;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]





