Abstract
Fatty acid synthesis in chloroplasts is regulated by light. The synthesis of malonyl-CoA, which is catalyzed by acetyl-CoA carboxylase (ACCase) and is the first committed step, is modulated by light/dark. Plants have ACCase in plastids and the cytosol. To determine the possible involvement of a redox cascade in light/dark modulation of ACCase, the effect of DTT, a known reductant of S-S bonds, was examined in vitro for the partially purified ACCase from pea plant. Only the plastidic ACCase was activated by DTT. This enzyme was activated in vitro more efficiently by reduced thioredoxin, which is a transducer of redox potential during illumination, than by DTT alone. Chloroplast thioredoxin-f activated the enzyme more efficiently than thioredoxin-m. The ACCase also was activated by thioredoxin reduced enzymatically with NADPH and NADP-thioredoxin reductase. These findings suggest that the reduction of ACCase is needed for activation of the enzyme, and a redox potential generated by photosynthesis is involved in its activation through thioredoxin as for enzymes of the reductive pentose phosphate cycle. The catalytic activity of ACCase was maximum at pH 8 and 2–5 mM Mg2+, indicating that light-produced changes in stromal pH and Mg2+ concentration modulate ACCase activity. These results suggest that light directly modulates a regulatory site of plastidic prokaryotic form of ACCase via a signal transduction pathway of a redox cascade and indirectly modulates its catalytic activity via stromal pH and Mg2+ concentration. A redox cascade is likely to link between light and fatty acid synthesis, resulting in coordination of fatty acid synthesis with photosynthesis.
Keywords: coordination with photosynthesis, malonyl-CoA synthesis, pea, redox cascade, signal transduction
Acetyl-CoA carboxylase (ACCase) regulates the rate of fatty acid synthesis in yeasts, animals, Escherichia coli, and plants (1, 2). In yeasts and animals, this enzyme activity is controlled by a variety of metabolites and by phosphorylation and dephosphorylation so that excess energy is stored in the form of fatty acids in response to the environmental conditions (1, 3). Such regulation has not yet been found in plants (4). Plant fatty acids are synthesized mainly in plastids, and a regulatory system different from that of other eukaryotes and characteristic of plastids may be involved. Regulation via redox is an important mechanism characteristic of chloroplasts (5). Enzyme activities of the reductive pentose phosphate cycle in chloroplasts are regulated via a redox cascade generated by light (5–9). In the light, electrons from photosystem I are shuttled through the electron transport chain to ferredoxin and are transferred to thioredoxins by ferredoxin-thioredoxin reductase, and then to target enzymes, reducing a disulfide bond(s) of the enzymes and changing their catalytic activities. Synthesis of certain proteins in chloroplasts also is regulated via a redox cascade (10). In the redox cascade, thioredoxin acts as a transducer of redox potential generated by the light reactions of photosynthesis, providing the chloroplasts with a mechanism to coordinate the activity of various components of photosynthesis to the presence or absence of light (5–9).
De novo fatty acid synthesis in chloroplasts increases in the light and decreases in the dark. The first committed step of this synthesis, catalyzed by ACCase, is the formation of malonyl-CoA. Isolated chloroplasts incorporate acetate into malonyl-CoA within minutes when exposed to light and the incorporation decreases when exposure ends (11). This pattern of change has been partly explained by changes in ACCase activity via the pH, Mg2+, and adenine nucleotide levels of the chloroplast stroma (2, 11–13). Fatty acid synthesis also may be regulated through a redox cascade, but evidence is lacking.
Recently we identified two forms of ACCase and showed that most plants examined so far have the prokaryotic form in plastids and the eukaryotic form in cytosol. Of the plants examined, only Gramineae have the eukaryotic form in both plastids and the cytosol (14–17). The eukaryotic form of the enzyme from several plants has been purified and characterized, but the prokaryotic form has not. Here we obtained the prokaryotic form of the enzyme partially purified from pea and examined its properties to find if a redox cascade was involved in malonyl-CoA synthesis. We examined the effects of DTT and reduced thioredoxin on plastidic prokaryotic ACCase activity, in addition to the effects of pH and Mg2+, and compared the effects with those on the eukaryotic form of ACCase.
MATERIALS AND METHODS
Reagents.
Thioredoxin from E. coli and the reduced form of dl-α-lipoic acid (dl-6,8 thioctic acid) were purchased from Sigma. The reduced form of E. coli thioredoxin was generally prepared by the incubation of 0.32 mM thioredoxin in 50 mM Tricine⋅KOH, pH 8, with 4 mM DTT at 30°C for 10 min, and then DTT was removed by centrifuging in a gel filtration column (Biospin 6, Bio-Rad). Recombinant spinach thioredoxins-f and -m, gifts from P. Schürmann (Université de Neuchâtel, Switzerland), were prepared as described (18). Thioredoxin reductase from E. coli was purchased from American Diagnostica (Greenwich, CT).
Partial Purification of Plastidic ACCase.
Pea plants (Pisum sativum cv. Alaska) were grown with a cycle of 16 hr of light and 8 hr of dark. Intact chloroplasts were isolated from leaves 9 days old with a Percoll gradient. The isolated chloroplasts were ruptured by a lysis buffer (50 mM Tricine⋅KOH, pH 8/1 mM EDTA/10 mM 2-mercaptoethanol/1 mM phenylmethylsulfonyl fluoride/1 mM benzamide/5 mM ɛ-amino-n-caproic acid) as described before (19). The lysed chloroplasts were centrifuged for 20 min at 50,000 × g. The supernatant fraction was precipitated with ammonium sulfate (50% saturation) and centrifuged. The precipitated proteins were dissolved and dialyzed against the lysis buffer. The material in the dialysis bag (about 50 mg of protein in 2 ml of lysis buffer) was applied to a Sephacryl S-300 column (1.6 × 60 cm, Pharmacia) and eluted with lysis buffer at a flow rate of 0.4 ml/min. The fractions were assayed for ACCase activity. The active fractions were precipitated with ammonium sulfate (50% saturation), and the suspension was divided into several portions. The ammonium sulfate precipitates were recovered by brief centrifugation and stored at −20°C until use. This fraction was free of cytosolic ACCase.
Partial Purification of Cytosolic ACCase.
Pea seedlings were grown in the dark for 5 days and in the light (under a white fluorescent lamp, about 10,000 lux) for 36 hr. Apical buds (3.5 g) were macerated with sand in 10 ml of lysis buffer and centrifuged at 100,000 × g for 30 min. Ammonium sulfate was added to 50% saturation to the supernatant. The precipitated protein was dissolved in 2.5 ml of lysis buffer and applied to a column of Sephacryl S-300 column as described above. The fractions with ACCase activity that contained protein with a molecular weight of 500,000 were collected and treated as described above. All of the ACCase in these fractions is known to be cytosolic (15).
Measurement of ACCase Activity.
The ammonium sulfate precipitates of the plastidic or the cytosolic ACCase fraction were dissolved in 50–100 μl of a 50 mM Tricine⋅KOH (pH 8) buffer containing 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamide, and 5 mM ɛ-amino-n-caproic acid, and desalted by centrifugation in a gel filtration column (Biospin 6, or Biospin 30, Bio-Rad). ACCase activity was measured as described elsewhere (14). In brief, 5 μl of protein solution containing 5–7 μg of protein in a total of 20 μl of a reaction mixture containing 50 mM Tricine⋅KOH (pH 8), 1 mM ATP, 2.5 mM MgCl2, 1 mM DTT, 50 mM KCl, 1.2 mM acetyl-CoA, and 3.4 mM NaH14CO3 (14.8 kBq) was incubated at 30°C for 20 min, and the reaction was stopped by the addition of 5 μl of 6 N HCl. Then 25 μl of the reaction mixture was applied to Whatman 3MM paper (1 × 2 cm). The paper was dried, and radioactivity was determined. When the enzyme was preincubated with thiol reagents at 30°C for 10 min, the reaction was started by the addition of Tricine⋅KOH (pH 8)/ATP/MgCl2/KCl/acetyl-CoA/NaH14CO3. The final concentration was the same as described above.
RESULTS
Plastidic ACCase Preparation.
Plastidic ACCase was purified from isolated chloroplasts by ammonium sulfate fractionation and gel filtration. This enzyme is a large complex with a molecular weight of 700,000 (14), separable from the contaminant cytosolic ACCase (500,000) and the major stromal protein, ribulose-bisphosphate carboxylase (550,000). The enzyme was purified 30-fold by gel filtration. Further attempts at purification by ion-exchange chromatography and affinity chromatography were unsuccessful, probably because the enzyme was labile and dissociable. The activity of the soluble enzyme was lost during storage at −20°C. The addition of a stabilizing agent such as glycerol or BSA did not stabilize activity. The activity of the ammonium sulfate precipitate stored at −20°C was stable for about 6 months. We stored the enzyme in this way and used it after desalting the precipitate on a gel filtration column. This enzyme solution was stable at 30°C for 5 hr in the presence of 1 mM DTT, but irreversibly lost 75% of its activity without DTT.
Effect of Thiol Reagents on Activity.
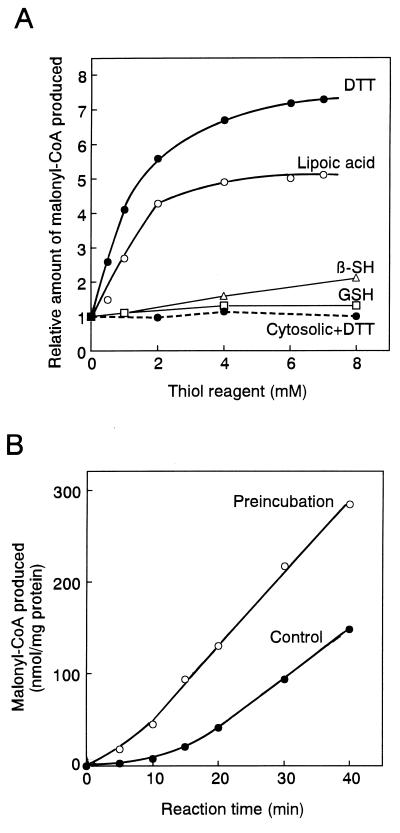
Fig. 1A shows the effects of four reducing agents on ACCase activity. The addition of the monothiol reducing agent 2-mercaptoethanol or reduced glutathione increased the plastidic ACCase activity slightly, but the addition of the dithiol reducing agent lipoic acid (thioctic acid) or DTT increased the activity severalfold. This result indicates that the enzyme is sensitive to redox state. To determine whether the requirement for DTT was specific for the plastidic prokaryotic ACCase, we examined the effect of DTT on cytosolic eukaryotic ACCase. The activity of the cytosolic ACCase from pea was not increased by DTT, indicating the requirement for DTT was specific for the plastidic ACCase. These results suggest that a redox-responsive regulatory site is present in the plastidic ACCase and reduction of an S-S bond(s) is needed for activation. The fact that dithiol reagents activated more efficiently than monothiols indicates the requirement of vicinal dithiol groups for the activation. This requirement is observed for the activation of chloroplast stromal enzymes by thioredoxin (5–9).
Figure 1.
Effects of thiol reagents on the ACCase activity. (A) ACCase activity was measured in the indicated concentration of a thiol reagent as described in Materials and Methods. Plastidic ACCase from pea, continuous lines; cytosolic ACCase from pea, dashed line. β-SH, 2-mercaptoethanol; GSH, reduced form of glutathione. Lipoic acid was dissolved into 50 mM Tricine⋅KOH (pH 8) containing 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamide, and 5 mM ɛ-amino-n-caproic acid at 20 mM, and 0–7 μl of 20 mM solution was added to 13-μl reaction mixture. The activity without any thiol reagent was 11 nmol/20 min per mg of protein for pea plastidic ACCase and 210 nmol/20 min per mg of protein for pea cytosolic ACCase. The activity is relative to that without a thiol reagent, taken to be 1. Results are the means of duplicate determinations that differed less than 10%. (B) The effects of preincubation of plastidic ACCase with DTT on activity were examined. Changes with time are shown. After the enzyme was incubated at 30°C for 10 min with 2 mM DTT, the reaction was started by the addition of buffer, ATP, MgCl2, KCl, acetyl-CoA, and NaH14CO3. The final concentrations of these reagents were the same as above. The final concentration of DTT was 1 mM.
Preincubation of the enzyme with DTT increased its activity (Fig. 1B), further evidence that a redox-responsive regulatory site in ACCase activates this enzyme. Such treatment did not affect the cytosolic ACCase activity (data not shown).
The control reaction in Fig. 1B showed a lag phase of about 10 min, and then the reaction proceeded almost linearly. This result suggested that at least 10 min was required for the activation of enzyme, although a clear distinction between activation and catalysis was difficult to estimate. Thereafter the reaction was started after preincubation of the ACCase with reducing agent for 10 min.
Effects of Thioredoxin on Activity.
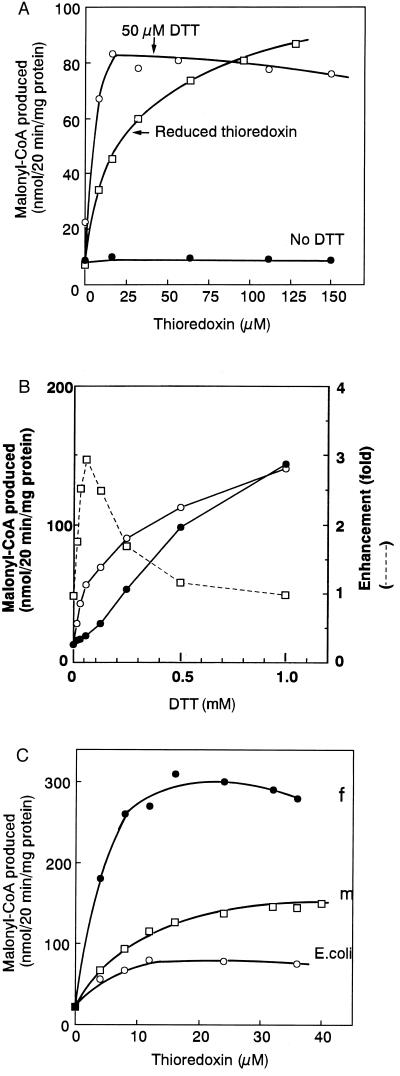
To gain further evidence on its mode of regulation, we determined the effect of reduced thioredoxin on plastidic ACCase (Fig. 2A). In this experiment, E. coli thioredoxin was used as in the original chloroplast experiments (20). Chloroplast thioredoxins are of low molecular weight (about 12,000) (21), and endogenous thioredoxins were not present in the partially purified enzyme preparation we used. As the concentration of stromal thioredoxin-m has been reported to be 100–200 μM (22), we examined the effect of thioredoxin in the range of 0–150 μM. Reduced thioredoxin was prepared by incubation with DTT, and excess DTT was removed by gel filtration. After incubation of the plastidic ACCase with the oxidized or reduced form of thioredoxin, the enzyme reaction was started. The addition of thioredoxin in its oxidized form did not increase the activity, but the addition of reduced thioredoxin increased the activity about 10-fold (Fig. 2A). The addition of 50 μM DTT to oxidized thioredoxin increased the activity. These results showed that reduced thioredoxin activates the plastidic form of ACCase in pea.
Figure 2.
Effects of reduced thioredoxin on plastidic ACCase activity. (A) Pea plastidic ACCase was preincubated with various concentrations of thioredoxin (oxidized form) in the presence (○) and absence (•) of 50 μM DTT or reduced thioredoxin (□) at 30°C for 10 min, and the reaction was started as in Fig. 1B. For no DTT, thioredoxin (oxidized form) was added. (B) Pea plastidic ACCase was preincubated with various concentrations of DTT in the presence (○) and absence (•) of 32 μM thioredoxin at 30°C for 10 min, and treated as in A. The final concentration of DTT was indicated. The activity in the presence of thioredoxin was divided by that in its absence at various DTT concentration, and the value obtained was plotted as enhancement (□). (C) Various concentrations of E. coli thioredoxin (○), spinach thioredoxin-f (•) or -m (□) were incubated with 50 μM DTT at 20°C for 10 min to reduce thioredoxin, and then the pea plastidic ACCase was added. After the mixture was further incubated at 30°C for 10 min, the enzyme reaction was started as in Fig. 1B. The final concentrations of thioredoxins were indicated.
Addition of various concentrations of DTT to preincubation mixtures in the presence or absence of a fixed amount of oxidized thioredoxin showed that activation was dependent on DTT concentration (Fig. 2B). Excess DTT (1 mM) directly activated the ACCase, and no effect of thioredoxin was observed. At a lower concentration, less than 1 mM DTT, the effect of thioredoxin increased with decreasing DTT concentrations. The activity enhancement of thioredoxin reached maximum at about 50 μM DTT (Fig. 2B), the concentration used above in Fig. 2A. We, therefore, used this concentration of DTT in our later studies with thioredoxin. The results suggest that DTT reduced the S-S bond of thioredoxin and that the reduced (dithiol) thioredoxin, having a higher affinity for the ACCase than DTT, reduced an S-S bond(s) and activated the enzyme.
To confirm the effect of reduced thioredoxin on activity further, we designed an experiment to reduce thioredoxin, not by DTT, but by an enzyme. Because E. coli NADPH-dependent thioredoxin reductase (NTR) reduces thioredoxin, one can supply reduced thioredoxin with NADPH in the presence of this enzyme (23). Simultaneous addition of NTR, NADPH, and thioredoxin to the preincubation mixture activated the ACCase about 5-fold (Table 1). This result showed the reduced thioredoxin was effective in activating the enzyme in the absence of DTT. Thus, thioredoxin, reduced either chemically with DTT or enzymatically with NTR, activated ACCase.
Table 1.
Effect of NADPH-dependent thioredoxin reductase on plastidic ACCase
| Additives | Malonyl-CoA produced, nmol/20 min per mg of protein |
|---|---|
| Control | 10 |
| DTT | 14.2 |
| DTT + thioredoxin | 58 |
| NADPH | 9.2 |
| NADPH + NTR | 8.6 |
| NADPH + thioredoxin | 10 |
| NADPH + thioredoxin + NTR | 53 |
Pea plastidic ACCase was incubated with the indicated additives for 10 min at 30°C (total volume 13 μl), and then the reaction was started as in Fig. 1B. The final concentrations of additives were: 50 μM DTT, 16 μM thioredoxin, 200 μM NADPH, and 0.2 μM NTR. Results are means of duplicate determinations that differed less than 10%.
Chloroplasts contain two thioredoxins, f and m. We, therefore, determined the relative effectiveness of these thioredoxins in activating the plastidic ACCase in the presence of 50 μM DTT (Fig. 2C). Recombinant spinach thioredoxins-f and -m were tested. In each case, increasing concentrations of thioredoxin enhanced ACCase activity in the range of 0–20 μM, and then the activity was constant. Under these conditions, both spinach thioredoxins-f and -m activated the enzyme more efficiently than E. coli thioredoxin. Of the two, thioredoxin-f activated the enzyme more efficiently than thioredoxin-m. Addition of thioredoxin-f stimulated ACCase activity about 14-fold over that of DTT alone. This result suggests that plastidic ACCase is activated preferentially by thioredoxin-f in vivo.
Effects of pH and Mg2+ on Activity.
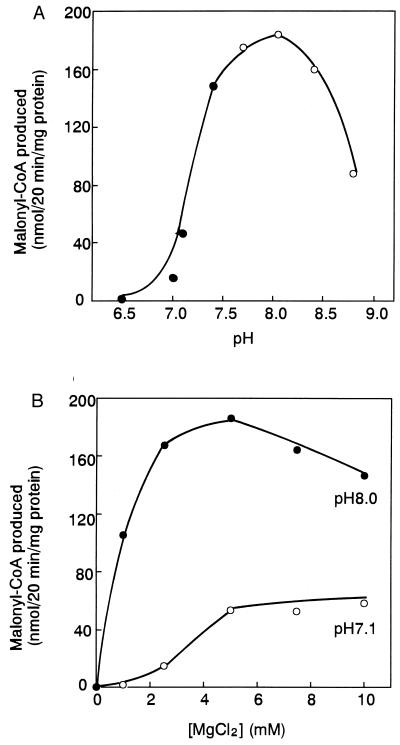
Fig. 3 shows the effects of pH and Mg2+ on the plastidic ACCase reaction. The reaction was started after preincubation of the enzyme with 1 mM DTT at pH 8.0. The enzyme showed maximal catalytic activity at about pH 8 with 2.5–5 mM Mg2+. A similar pH profile was obtained in the presence of 4 mM DTT (data not shown). The addition of thioredoxin to preincubation mixture at pH 8 did not change the Mg2+ profile, although twice as much malonyl-CoA was formed (data not shown). In the presence of 2.5 mM Mg2+, the activity at pH 8.0 was 10-fold that at pH 7.0. During illumination, increases in pH from 7 to 8 and in Mg2+ from 1 to 3 mM have been reported for the chloroplast stroma (24). The activity of the ACCase at pH 8.0 and 2.5 mM Mg2+ was 90-fold that at pH 7 and 1 mM Mg2+, suggesting that stromal ACCase could be activated very strongly by those changes in the light. Changes in stromal pH and Mg2+ likely affected the catalytic reaction of ACCase in vivo, as suggested by earlier observations (2). Effects of pH and Mg2+ on plastidic prokaryotic form of ACCase are reminiscent of the effects observed with the thioredoxin-linked enzymes of the reductive pentose phosphate cycle (5, 6).
Figure 3.
Effects of pH and Mg2+ on plastidic ACCase activity. (A) Pea plastidic ACCase was preincubated with 2 mM DTT at pH 8.0 at 30°C for 10 min, and then the reaction was started as in Fig. 1B except that different pHs were used. The final concentration of DTT was 1 mM. •, Mops⋅KOH buffer; ○, Tricine⋅KOH buffer. (B) After incubation of the enzyme with DTT as in A, the reaction was started as in Fig. 1B except that different concentrations of MgCl2 and pH were used, with Mops⋅KOH buffer at pH 7.1 and Tricine⋅KOH buffer at pH 8.0.
Comparison of Activity in Two Sets of Conditions.
Table 2 shows the ACCase activity in vitro under putative stromal conditions in the light and in the dark. Under the conditions prevailing in the light, the activity of plastidic ACCase from pea was 219-fold greater than in the dark; the corresponding change for cytosolic ACCase was only 5.5-fold. These results indicate that only the plastidic (prokaryotic) form of ACCase was significantly affected in vitro by changes in redox, pH, and Mg2+ concentration. The cytosolic eukaryotic form of ACCase from pea has been purified (25). As seen here, the enzyme has a broad pH optimum around pH 8.0–8.5, and a broad optimum Mg2+ concentration of 2–5 mM. This enzyme was not activated by DTT (Fig. 1A), and only marginally by changes in the pH and the Mg2+ concentration.
Table 2.
ACCase activity under simulated light and dark conditions
| Conditions | Malonyl-CoA produced, nmol/20 min per mg of protein
|
|
|---|---|---|
| Pea plastids | Pea cytosol | |
| “Dark” at pH 7.0 and with 1 mM Mg2+ but 0 mM DTT | 0.8 | 45 |
| “Light” at pH 8.0 and with 2.5 mM Mg2+ and 1 mM DTT | 175 | 250 |
| “Light”-stimulation | 219 | 5.5 |
After the enzyme was preincubated with 32 μM E coli thioredoxin (final concentration) and the indicated concentration of DTT under two sets of conditions at 30°C for 10 min, the reaction was started as in Fig. 1B. Results are the means of duplicate determinations that differed less than 10%.
DISCUSSION
The synthesis of one molecule of palmitic acid requires 14 molecules of NADPH and seven molecules of ATP. In chloroplasts the NADPH and ATP are produced by the light reactions of photosynthesis. A link between the light reactions of photosynthesis and fatty acid synthesis is needed to balance the production and the consumption of these metabolites during illumination. One possible way to link the two reactions is via a redox cascade using thioredoxin light-induced changes in stromal pH and Mg2+ concentration. The findings presented above suggest that ACCase, the first step in fatty acid biosynthesis, can be regulated by these means analogous to the thioredoxin-linked enzymes of the reductive pentose phosphate cycle (5–9). The plastidic form of ACCase was activated by reduced thioredoxin or a dithiol such as DTT or reduced lipoic acid. The activity of the enzyme also was found to be greatest at the pH (8.0) and Mg2+ concentration (2.5 mM) prevailing in the light. Thus, light may directly modulate a regulatory site of plastidic ACCase via a redox cascade and indirectly modulate its catalytic activity via changes in stromal pH and Mg2+ concentration.
A growing body of evidence shows that reducing power generated by the light reactions of photosynthesis reduces thioredoxin, which, in turn, reduces target enzymes of a number of chloroplast processes. The reduced (dithiol) form of the enzyme(s) is reoxidized by oxygen produced concomitantly. Hence, in the light, the target enzyme is continuously part of an oxidation-reduction cycle, but in the dark, cycling stops and the ratio of reduced to oxidized enzyme shifts so that the oxidized form is mainly present. Enzymes of the reductive pentose phosphate cycle, such as fructose 1,6-bisphosphatase (26), sedoheptulose 1,7-bisphosphatase (27), NADP-glyceraldehyde 3-phosphate dehydrogenase (28), and phosphoribulokinase (29), are regulated in this way by the ferredoxin-thioredoxin system. The other type of regulatory agents contributed by light, namely, changes in the stromal pH and Mg2+ also modulates enzyme activities. Our experiments showed that plastidic ACCase is likely regulated in a similar way. The reductive pentose phosphate cycle is the main biochemical pathway for the conversion of atmospheric CO2 to carbohydrates. By the simultaneous activation of key enzymes of this cycle and a pace-maker enzyme of fatty acid synthesis, light can be used for the production of reserve materials and other important chloroplast compounds.
Two thioredoxins, f and m, are found in chloroplasts. The sequence identity with E. coli thioredoxin is 24% for thioredoxin-f and 47% for thioredoxin-m. Although sharing the unique active center sequence—Cys-Gly-Pro-Cys—(21, 30), the two thioredoxins have a different phylogenetic history (6, 30–32). Thioredoxin-f selectively activates four of the five regulatory enzymes of the reductive pentose phosphate cycle, and thioredoxin-m activates NADP+-malate dehydrogenase (9, 33). Both thioredoxins are effective in activation of chloroplast ATP synthase (CF1-ATPase) (9). The plastidic ACCase was more effectively activated by thioredoxin-f than by thioredoxin-m. In the case of fructose-1,6-bisphosphatase, which is linked to thioredoxin-f, the Mg2+ required by the enzyme activation decreases in the presence of reduced thioredoxin (26). However, the Mg2+ requirement for the plastidic ACCase (Fig. 3B) did not change under these conditions, suggesting that ACCase regulation differs from that of the fructose-1,6-bisphosphatase. Further experiments are needed for characterization of the interaction of thioredoxin-f with ACCase.
To date, attempts to discover if redox modulation is linked to fatty acid synthesis have failed. Earlier experiments designed to show a stimulatory effect of dithiols with isolated chloroplasts or chloroplast extracts (11, 12) revealed that fatty acid synthesis was inhibited, not increased, by the addition of DTT. Most likely, DTT affected a number of components, resulting in observations difficult to interpret. For example, when DTT activates photosynthetic CO2 assimilation, competition for ATP results between fatty acid synthesis and the reductive pentose cycle. In addition, DTT undergoes nonenzymic acetylation with acyl-CoA thioesters (34), possibly causing acetyl-CoA deficiency as the fatty acid intermediate is formed. A simple assay seemed, therefore, to be needed to demonstrate the effect of DTT on ACCase activity. Using the partially purified enzyme, we characterized in vitro the ACCase reaction in this report and showed that increased concentrations of DTT activated the ACCase from pea plastids. Acetyl-CoA deficiency, therefore, was not caused by nonenzymic acylation with DTT (34) under the used conditions, and the assay was sufficiently reliable to show effect of a DTT on the enzyme.
Reduced thioredoxin was effective, probably by reducing the S-S bond of the regulatory site of the plastid ACCase. Several potential cysteine residues in the enzyme could be targeted by thioredoxin. The prokaryotic form of ACCase consists of four polypeptides, biotin carboxylase (BC), biotin carboxyl carrier protein (BCCP), transcarboxylase α subunit (TC-α), and transcarboxylase β subunit (TC-β). In plants, BC, BCCP, and TC-α are encoded in the nuclear (35–37), and TC-β in the chloroplast genome (14). The cDNAs and DNAs for these subunits have been cloned and sequenced from various plants. The number of conserved cysteine residues in BC, BCCP, TC-α, and TC-β are 3, 1, 2, and 5, respectively. The nuclear-encoded eukaryotic form of ACCase consists of a large single polypeptide containing BC, BCCP, and TC domains (38–40). One of the cysteine residues in BC is conserved in the BC domain of the eukaryotic form. The other cysteine residues conserved in plastidic ACCase are not found in eukaryotic form of ACCase. Therefore, one or more of the above-mentioned cysteines are potential regulatory residues. At present we do not know whether the prokaryotic form other than the chloroplastic one is redox-regulated. The redox regulation of ACCase may be a characteristic of plastidic prokaryotic form. More work is needed to identify the regulatory cysteine residues.
The amount of ACCase in leaf cells is strictly controlled during development (41), probably at the level of transcription. Posttranslationally, the enzyme activity seems to be mainly controlled by light and the physiological changes it affects in the plastid.
Acknowledgments
We thank P. Schürmann for spinach thioredoxins-f and -m, B. B. Buchanan, K. Wada, M. Hirose, and K. Asada for discussions, and H. Iguchi for technical assistance. This work was supported by grants from the Japanese Ministry of Education, Science, and Culture (The Molecular Basis of Flexible Organ Plan in Plants, No. 06278102), and from the Japan Society for the Promotion of Science (Research for the Future Program, JSPS-RTFT 96L006012). A.K. is a Japan Society for the Promotion of Science Research Associate.
ABBREVIATIONS
- ACCase
acetyl-CoA carboxylase
- BC
biotin carboxylase
References
- 1.Wakil S J, Stoops J K, Joshi V C. Annu Rev Biochem. 1983;52:537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- 2.Harwood J L. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:101–138. [Google Scholar]
- 3.Woods A, Munday M R, Scott J, Yang X, Carlson M, Carling D. J Biol Chem. 1994;269:19509–19515. [PubMed] [Google Scholar]
- 4.Ohlrogge J, Browse J. Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan B B. Annu Rev Plant Physiol. 1980;31:341–374. [Google Scholar]
- 6.Buchanan B B. Arch Biochem Biophys. 1991;288:1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- 7.Scheibe R. Plant Physiol. 1991;96:1–3. doi: 10.1104/pp.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolosiuk R A, Ballicora M A, Hagelin K. FASEB J. 1993;7:622–637. doi: 10.1096/fasebj.7.8.8500687. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan B B, Schürmann P, Decottignies P, Lozano R M. Arch Biochem Biophys. 1994;314:257–260. doi: 10.1006/abbi.1994.1439. [DOI] [PubMed] [Google Scholar]
- 10.Danon A, Mayfield S P. Science. 1994;266:1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- 11.Sauer A, Heise K-P. Plant Physiol. 1983;73:11–15. doi: 10.1104/pp.73.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauer A, Heise K-P. Z Naturforsch. 1984;39C:268–275. [Google Scholar]
- 13.Eastwell K C, Stumpf P K. Plant Physiol. 1983;72:50–55. doi: 10.1104/pp.72.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki Y, Hakamada K, Suama Y, Nagano Y, Furusawa I, Matsuno R. J Biol Chem. 1993;268:25118–25123. [PubMed] [Google Scholar]
- 15.Konishi T, Sasaki Y. Proc Natl Acad Sci USA. 1994;91:3598–3601. doi: 10.1073/pnas.91.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki Y, Konishi T, Nagano Y. Plant Physiol. 1995;108:445–449. doi: 10.1104/pp.108.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konishi T, Shinohara K, Yamada K, Sasaki Y. Plant Cell Physiol. 1996;37:117–122. doi: 10.1093/oxfordjournals.pcp.a028920. [DOI] [PubMed] [Google Scholar]
- 18.Schürmann P. Methods Enzymol. 1995;252:274–283. doi: 10.1016/0076-6879(95)52030-9. [DOI] [PubMed] [Google Scholar]
- 19.Orozco M, Jr, Mullet J E, Hanley-Bowdoin L, Chua N H. Methods Enzymol. 1986;118:232–253. doi: 10.1016/0076-6879(86)18076-1. [DOI] [PubMed] [Google Scholar]
- 20.Wolosiuk R A, Buchanan B B. Nature (London) 1977;266:565–567. [Google Scholar]
- 21.Holmgren A. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 22.Scheibe R. FEBS Lett. 1981;133:301–304. [Google Scholar]
- 23.Holmgren A. Methods Enzymol. 1984;107:295–300. doi: 10.1016/0076-6879(84)07019-1. [DOI] [PubMed] [Google Scholar]
- 24.Portis A R, Jr, Heldt H W. Biochim Biophys Acta. 1976;449:434–446. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- 25.Bettey M, Ireland R J, Smith A M. J Plant Physiol. 1992;140:513–520. [Google Scholar]
- 26.Schürmann P, Wolosiuk R A. Biochim Biophys Acta. 1978;522:130–138. doi: 10.1016/0005-2744(78)90329-7. [DOI] [PubMed] [Google Scholar]
- 27.Breazeale V D, Buchanan B B, Wolosiuk R A. Z Naturforsch. 1978;33C:521–528. [Google Scholar]
- 28.Wolosiuk R A, Buchanan B B. Plant Physiol. 1978;61:669–671. doi: 10.1104/pp.61.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolosiuk R A, Buchanan B B. Arch Biochem Biophys. 1978;189:97–101. doi: 10.1016/0003-9861(78)90119-4. [DOI] [PubMed] [Google Scholar]
- 30.Wedel N, Clausmeyer S, Herrmann R G, Gardet-Salvi L, Schürmann P. Plant Mol Biol. 1992;18:527–533. doi: 10.1007/BF00040668. [DOI] [PubMed] [Google Scholar]
- 31.Hartman H, Syvanen M, Buchanan B B. Mol Biol Evol. 1990;7:247–254. doi: 10.1093/oxfordjournals.molbev.a040602. [DOI] [PubMed] [Google Scholar]
- 32.Sahrawy M, Hecht V, Lopez-Jaramillo J, Chueca A, Chartier Y, Meyer Y. J Mol Evol. 1996;42:422–431. doi: 10.1007/BF02498636. [DOI] [PubMed] [Google Scholar]
- 33.Scheibe R. Physiol Plant. 1987;71:393–400. [Google Scholar]
- 34.Stokes G B, Stumpf P K. Arch Biochem Biophys. 1974;162:638–648. doi: 10.1016/0003-9861(74)90226-4. [DOI] [PubMed] [Google Scholar]
- 35.Shorrosh B S, Roesler K R, Shintani D, Van de Loo F, Ohlrogge J B. Plant Physiol. 1995;108:805–812. doi: 10.1104/pp.108.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi J K, Yu F, Wurtele E S, Nikolau B J. Plant Physiol. 1995;109:615–629. doi: 10.1104/pp.109.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shorrosh B S, Savage L J, Soll T, Ohlrogge J B. Plant J. 1996;10:261–268. doi: 10.1046/j.1365-313x.1996.10020261.x. [DOI] [PubMed] [Google Scholar]
- 38.Shorrosh B S, Dixon R A, Ohlrogge J B. Proc Natl Acad Sci USA. 1994;91:4323–4327. doi: 10.1073/pnas.91.10.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gornicki P, Podkowinski J, Scappino L A, DiMaio J, Haselkorn R. Proc Natl Acad Sci USA. 1994;91:6860–6864. doi: 10.1073/pnas.91.15.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yanai Y, Kawasaki T, Shimada H, Wurtele E S, Nikolau B J, Ichikawa N. Plant Cell Physiol. 1995;36:779–787. doi: 10.1093/oxfordjournals.pcp.a078822. [DOI] [PubMed] [Google Scholar]
- 41.Konishi T, Kamoi T, Matsuno R, Sasaki Y. Plant Cell Physiol. 1996;37:1197–1200. [Google Scholar]