Abstract
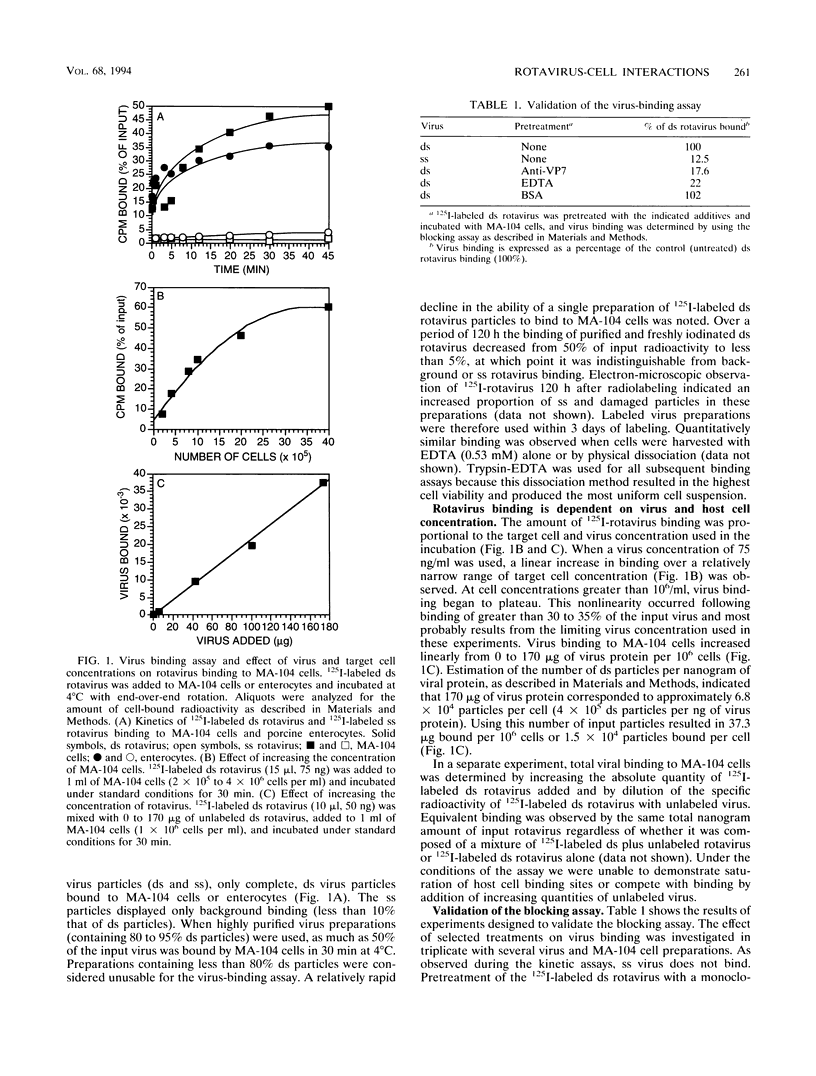
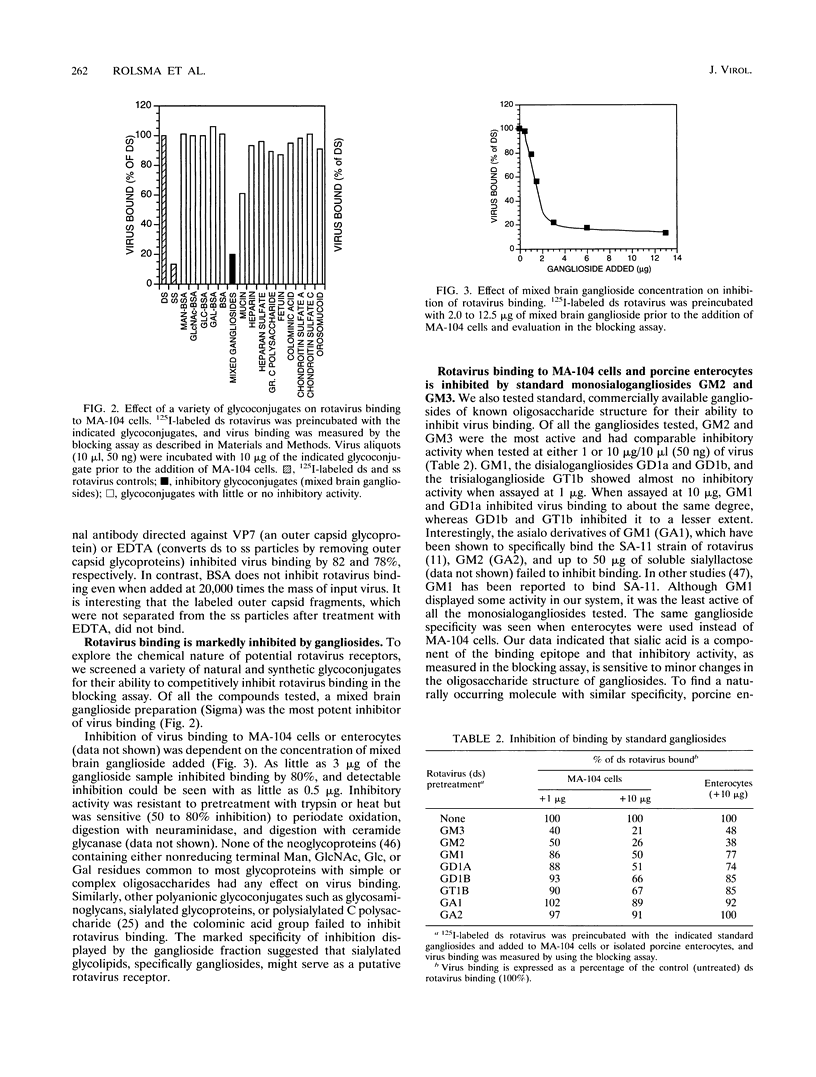
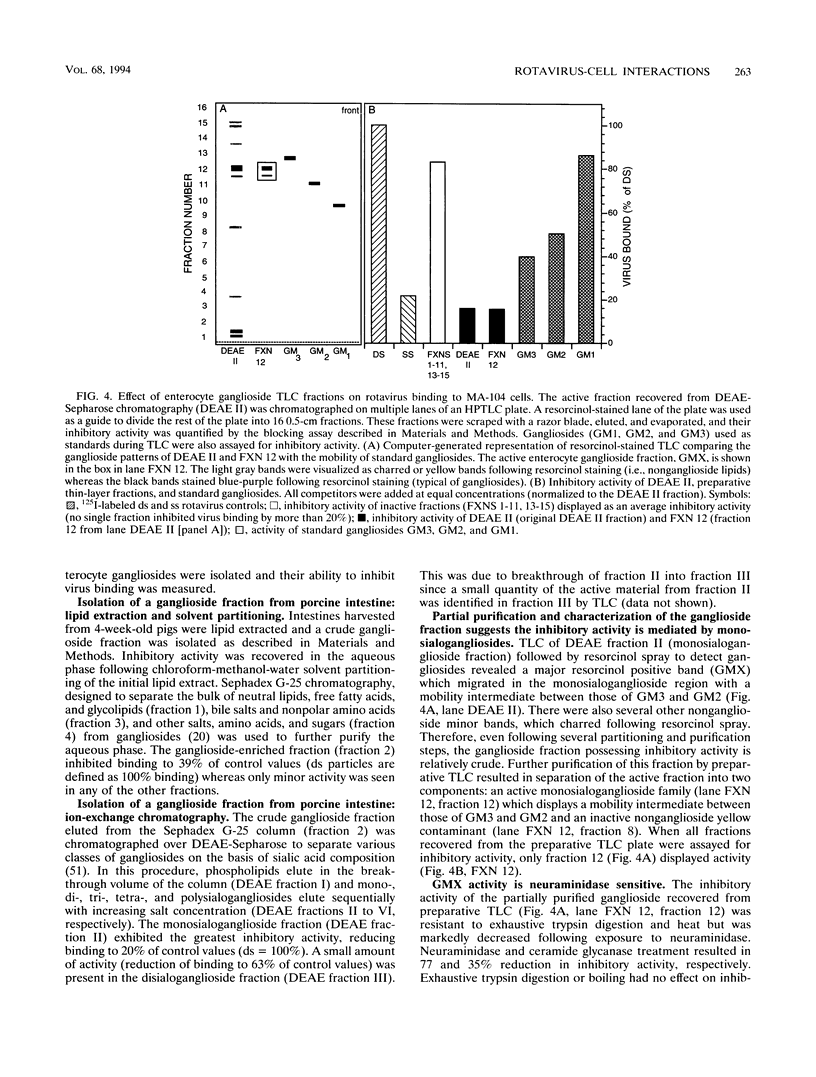
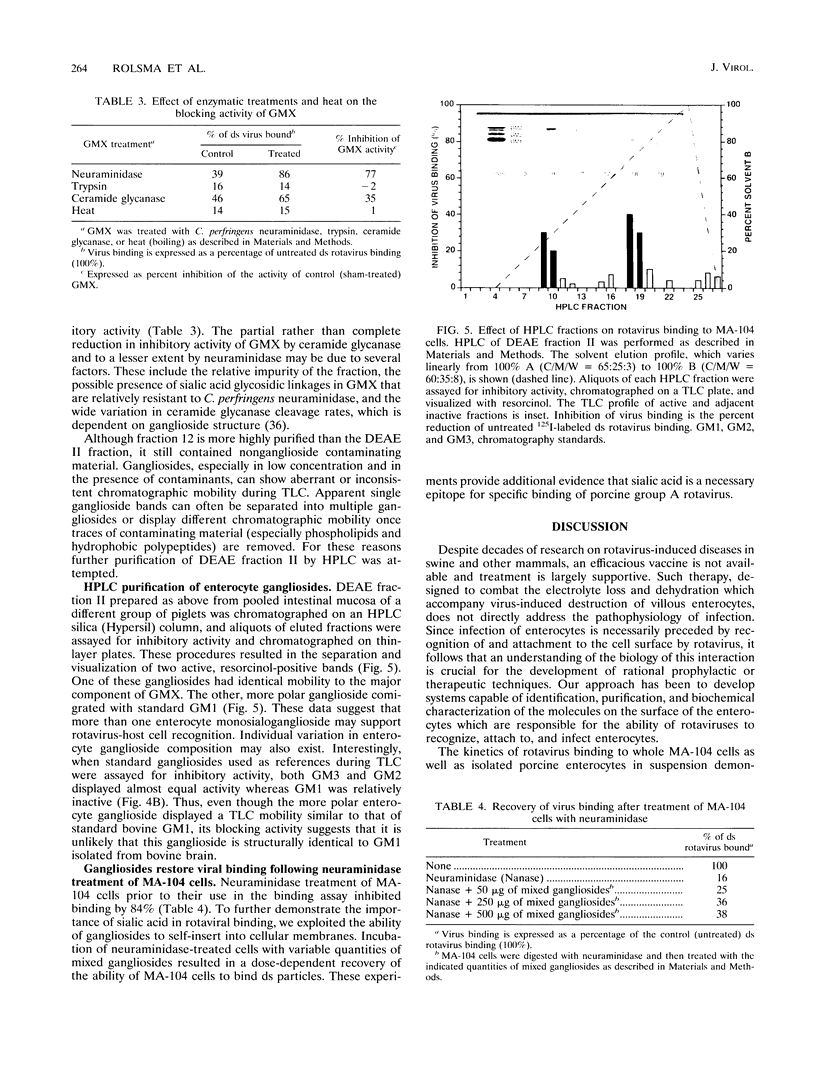
A virus-host cell-binding assay was developed and used to investigate specific binding between group A porcine rotavirus and MA-104 cells or porcine enterocytes. A variety of glycoconjugates and cellular components were screened for their ability to block rotavirus binding to cells. During these experiments a crude ganglioside mixture was observed to specifically block rotavirus binding. On the basis of these results, enterocytes were harvested from susceptible piglets and a polar lipid fraction was isolated by solvent extraction and partitioning. Throughout subsequent purification of this fraction by Sephadex partition, ion-exchange, silicic acid, and thin-layer chromatography, blocking activity behaved as a monosialoganglioside (GMX) that displayed a thin-layer chromatographic mobility between those of GM2 and GM3. The blocking activity of GMX was inhibited by treatment with neuraminidase and ceramide glycanase but not by treatment with protease or heat (100 degrees C). Further purification of GMX by high-pressure liquid chromatography resulted in the resolution of two monosialogangliosides, GMX and a band which comigrated with GM1 on thin-layer chromatography. These data suggest that a cell surface monosialoganglioside or family of monosialogangliosides may function as an in vivo relevant receptor for group A porcine rotavirus and that sialic acid is a required epitope for virus-binding activity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. M., Baylor M. R., Chen C., Mackow E. M., Bremont M., Greenberg H. B. Liposome-mediated transfection of intact viral particles reveals that plasma membrane penetration determines permissivity of tissue culture cells to rotavirus. J Clin Invest. 1992 Dec;90(6):2313–2320. doi: 10.1172/JCI116119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. M., Mackow E. R., Greenberg H. B. Identification and partial characterization of a rhesus rotavirus binding glycoprotein on murine enterocytes. Virology. 1991 Aug;183(2):602–610. doi: 10.1016/0042-6822(91)90989-o. [DOI] [PubMed] [Google Scholar]
- Bern C., Martines J., de Zoysa I., Glass R. I. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull World Health Organ. 1992;70(6):705–714. [PMC free article] [PubMed] [Google Scholar]
- Blackburn C. C., Swank-Hill P., Schnaar R. L. Gangliosides support neural retina cell adhesion. J Biol Chem. 1986 Feb 25;261(6):2873–2881. [PubMed] [Google Scholar]
- Blacklow N. R., Greenberg H. B. Viral gastroenteritis. N Engl J Med. 1991 Jul 25;325(4):252–264. doi: 10.1056/NEJM199107253250406. [DOI] [PubMed] [Google Scholar]
- Bohl E. H. Rotaviral diarrhea in pigs: brief review. J Am Vet Med Assoc. 1979 Mar 15;174(6):613–615. [PubMed] [Google Scholar]
- Cohen M. B. Etiology and mechanisms of acute infectious diarrhea in infants in the United States. J Pediatr. 1991 Apr;118(4 Pt 2):S34–S39. doi: 10.1016/s0022-3476(05)81423-4. [DOI] [PubMed] [Google Scholar]
- Feizi T. Carbohydrate differentiation antigens: probable ligands for cell adhesion molecules. Trends Biochem Sci. 1991 Mar;16(3):84–86. doi: 10.1016/0968-0004(91)90038-w. [DOI] [PubMed] [Google Scholar]
- Fukudome K., Yoshie O., Konno T. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology. 1989 Sep;172(1):196–205. doi: 10.1016/0042-6822(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Glick G. D., Toogood P. L., Wiley D. C., Skehel J. J., Knowles J. R. Ligand recognition by influenza virus. The binding of bivalent sialosides. J Biol Chem. 1991 Dec 15;266(35):23660–23669. [PubMed] [Google Scholar]
- Gottfries J., Davidsson P., Månsson J. E., Svennerholm L. High-performance liquid chromatographic separation of gangliosides, combined with immunological detection and fast atom bombardment mass spectrometric characterization. J Chromatogr. 1989 May 30;490(2):263–274. doi: 10.1016/s0378-4347(00)82784-x. [DOI] [PubMed] [Google Scholar]
- Hardy M. R., Townsend R. R., Parkhurst S. M., Lee Y. C. Different modes of ligand binding to the hepatic galactose/N-acetylgalactosamine lectin on the surface of rabbit hepatocytes. Biochemistry. 1985 Jan 1;24(1):22–28. doi: 10.1021/bi00322a004. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm L., Elwing H., Fredman P., Strannegård O. Sendai virus receptor: proposed recognition structure based on binding to plastic-adsorbed gangliosides. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1947–1950. doi: 10.1073/pnas.77.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaljot K. T., Shaw R. D., Rubin D. H., Greenberg H. B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988 Apr;62(4):1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Keljo D. J., Smith A. K. Characterization of binding of simian rotavirus SA-11 to cultured epithelial cells. J Pediatr Gastroenterol Nutr. 1988 Mar-Apr;7(2):249–256. doi: 10.1097/00005176-198803000-00015. [DOI] [PubMed] [Google Scholar]
- Knill-Jones R. P., Newman B. J., Spence A. A. Anesthetic practice and pregnancy. Controlled survey of male anaesthetists in the United Kingdom. Lancet. 1975 Oct 25;2(7939):807–809. [PubMed] [Google Scholar]
- Lecce J. G., King M. W., Dorsey W. E. Rearing regimen producing piglet diarrhea (rotavirus) and its relevance to acute infantile diarrhea. Science. 1978 Feb 17;199(4330):776–778. doi: 10.1126/science.203032. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C., Dunne F. T., Jonssen E. K. Studies on the meningococcal polysaccharides. II. Composition and chemical properties of the group B and group C polysaccharide. J Biol Chem. 1971 Aug 10;246(15):4703–4712. [PubMed] [Google Scholar]
- Méndez E., Arias C. F., López S. Binding to sialic acids is not an essential step for the entry of animal rotaviruses to epithelial cells in culture. J Virol. 1993 Sep;67(9):5253–5259. doi: 10.1128/jvi.67.9.5253-5259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi P., Charpilienne A., Cohen J. Interaction of rotavirus particles with liposomes. J Virol. 1992 Jun;66(6):3363–3367. doi: 10.1128/jvi.66.6.3363-3367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruchuri D. K., Seifert H. S., Ajioka R. S., Karlsson K. A., So M. Identification and characterization of a Neisseria gonorrhoeae gene encoding a glycolipid-binding adhesin. Proc Natl Acad Sci U S A. 1990 Jan;87(1):333–337. doi: 10.1073/pnas.87.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R. W., Choi A. H., Lee P. W. The alpha-anomeric form of sialic acid is the minimal receptor determinant recognized by reovirus. Virology. 1989 Sep;172(1):382–385. doi: 10.1016/0042-6822(89)90146-3. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991 Sep 6;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Powell L. D., Hart G. W. Quantitation of picomole levels of N-acetyl- and N-glycolylneuraminic acids by a HPLC-adaptation of the thiobarbituric acid assay. Anal Biochem. 1986 Aug 15;157(1):179–185. doi: 10.1016/0003-2697(86)90211-3. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Burns J. W., Marietta E., Estes M. K., Chiu W. Localization of VP4 neutralization sites in rotavirus by three-dimensional cryo-electron microscopy. Nature. 1990 Feb 1;343(6257):476–479. doi: 10.1038/343476a0. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A., Nelson W. L., Glode M. P., Triffon T. C., Kogut S. J., Yolken R. H., Hernandez J. A., Levin M. J. Neonatal rotavirus-associated necrotizing enterocolitis: case control study and prospective surveillance during an outbreak. J Pediatr. 1988 Jan;112(1):87–93. doi: 10.1016/S0022-3476(88)80128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Jessell T. M. Cell adhesion molecules in vertebrate neural development. Physiol Rev. 1988 Jul;68(3):819–857. doi: 10.1152/physrev.1988.68.3.819. [DOI] [PubMed] [Google Scholar]
- Saulsbury F. T., Winkelstein J. A., Yolken R. H. Chronic rotavirus infection in immunodeficiency. J Pediatr. 1980 Jul;97(1):61–65. doi: 10.1016/s0022-3476(80)80131-4. [DOI] [PubMed] [Google Scholar]
- Schnaar R. L., Brandley B. K., Needham L. K., Swank-Hill P., Blackburn C. C. Adhesion of eukaryotic cells to immobilized carbohydrates. Methods Enzymol. 1989;179:542–558. doi: 10.1016/0076-6879(89)79153-9. [DOI] [PubMed] [Google Scholar]
- Schnaar R. L. Complex carbohydrates in drug development. Adv Pharmacol. 1992;23:35–84. doi: 10.1016/S1054-3589(08)60962-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar R. L. Glycosphingolipids in cell surface recognition. Glycobiology. 1991 Nov;1(5):477–485. doi: 10.1093/glycob/1.5.477. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Lasky L. A. Cell adhesion. Sticky sugars for selectins. Nature. 1991 Jan 17;349(6306):196–197. doi: 10.1038/349196a0. [DOI] [PubMed] [Google Scholar]
- Srnka C. A., Tiemeyer M., Gilbert J. H., Moreland M., Schweingruber H., de Lappe B. W., James P. G., Gant T., Willoughby R. E., Yolken R. H. Cell surface ligands for rotavirus: mouse intestinal glycolipids and synthetic carbohydrate analogs. Virology. 1992 Oct;190(2):794–805. doi: 10.1016/0042-6822(92)90917-e. [DOI] [PubMed] [Google Scholar]
- Stowell C. P., Lee V. C. Neoglycoproteins: the preparation and application of synthetic glycoproteins. Adv Carbohydr Chem Biochem. 1980;37:225–281. doi: 10.1016/s0065-2318(08)60022-0. [DOI] [PubMed] [Google Scholar]
- Superti F., Donelli G. Gangliosides as binding sites in SA-11 rotavirus infection of LLC-MK2 cells. J Gen Virol. 1991 Oct;72(Pt 10):2467–2474. doi: 10.1099/0022-1317-72-10-2467. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kitaoka S., Konno T., Sato T., Ishida N. Two modes of human rotavirus entry into MA 104 cells. Arch Virol. 1985;85(1-2):25–34. doi: 10.1007/BF01317003. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kitaoka S., Sato T., Konno T., Iwasaki Y., Numazaki Y., Ishida N. Further investigation on the mode of entry of human rotavirus into cells. Arch Virol. 1986;91(1-2):135–144. doi: 10.1007/BF01316734. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Suzuki T., Matsunaga M., Matsumoto M. Gangliosides as paramyxovirus receptor. Structural requirement of sialo-oligosaccharides in receptors for hemagglutinating virus of Japan (Sendai virus) and Newcastle disease virus. J Biochem. 1985 Apr;97(4):1189–1199. doi: 10.1093/oxfordjournals.jbchem.a135164. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980 Jan 18;617(1):97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- Svensson L. Group C rotavirus requires sialic acid for erythrocyte and cell receptor binding. J Virol. 1992 Sep;66(9):5582–5585. doi: 10.1128/jvi.66.9.5582-5585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeyer M., Swiedler S. J., Ishihara M., Moreland M., Schweingruber H., Hirtzer P., Brandley B. K. Carbohydrate ligands for endothelial-leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1138–1142. doi: 10.1073/pnas.88.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasak R., Luytjes W., Spaan W., Palese P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4526–4529. doi: 10.1073/pnas.85.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman P. M. Profile of a mammalian sperm receptor. Development. 1990 Jan;108(1):1–17. doi: 10.1242/dev.108.Supplement.1. [DOI] [PubMed] [Google Scholar]
- Weigel P. H., Schnaar R. L., Kuhlenschmidt M. S., Schmell E., Lee R. T., Lee Y. C., Roseman S. Adhesion of hepatocytes to immobilized sugars. A threshold phenomenon. J Biol Chem. 1979 Nov 10;254(21):10830–10838. [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Holmes K. V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Snyder S. W., Frana M. F., Holmes K. V. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J Virol. 1990 Aug;64(8):3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby R. E., Yolken R. H., Schnaar R. L. Rotaviruses specifically bind to the neutral glycosphingolipid asialo-GM1. J Virol. 1990 Oct;64(10):4830–4835. doi: 10.1128/jvi.64.10.4830-4835.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M., Dryden K. A., Olson N. H., Greenberg H. B., Baker T. S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J Cell Biol. 1990 Jun;110(6):2133–2144. doi: 10.1083/jcb.110.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Bishop C. A., Townsend T. R., Bolyard E. A., Bartlett J., Santos G. W., Saral R. Infectious gastroenteritis in bone-marrow-transplant recipients. N Engl J Med. 1982 Apr 29;306(17):1010–1012. doi: 10.1056/NEJM198204293061701. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Willoughby R., Wee S. B., Miskuff R., Vonderfecht S. Sialic acid glycoproteins inhibit in vitro and in vivo replication of rotaviruses. J Clin Invest. 1987 Jan;79(1):148–154. doi: 10.1172/JCI112775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Lee A. M., Bassler B. L., Roseman S. Chitin utilization by marine bacteria. A physiological function for bacterial adhesion to immobilized carbohydrates. J Biol Chem. 1991 Dec 25;266(36):24260–24267. [PubMed] [Google Scholar]




