Abstract
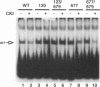
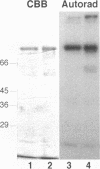
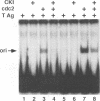
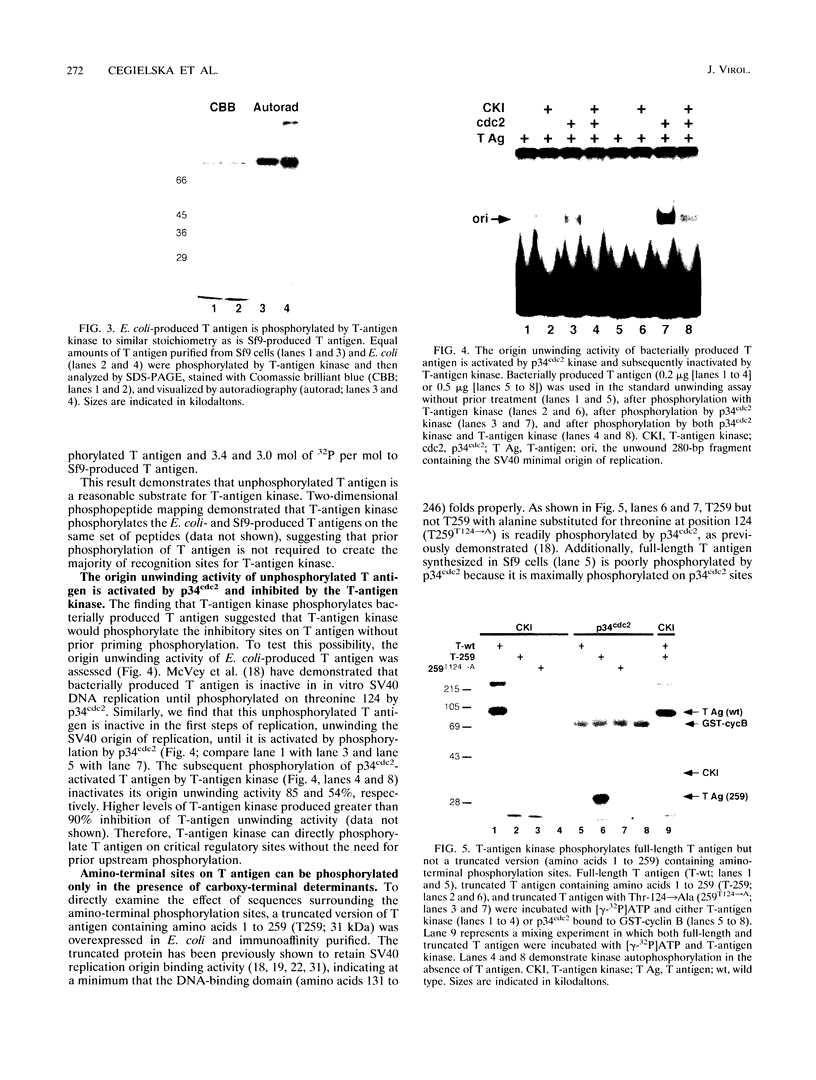
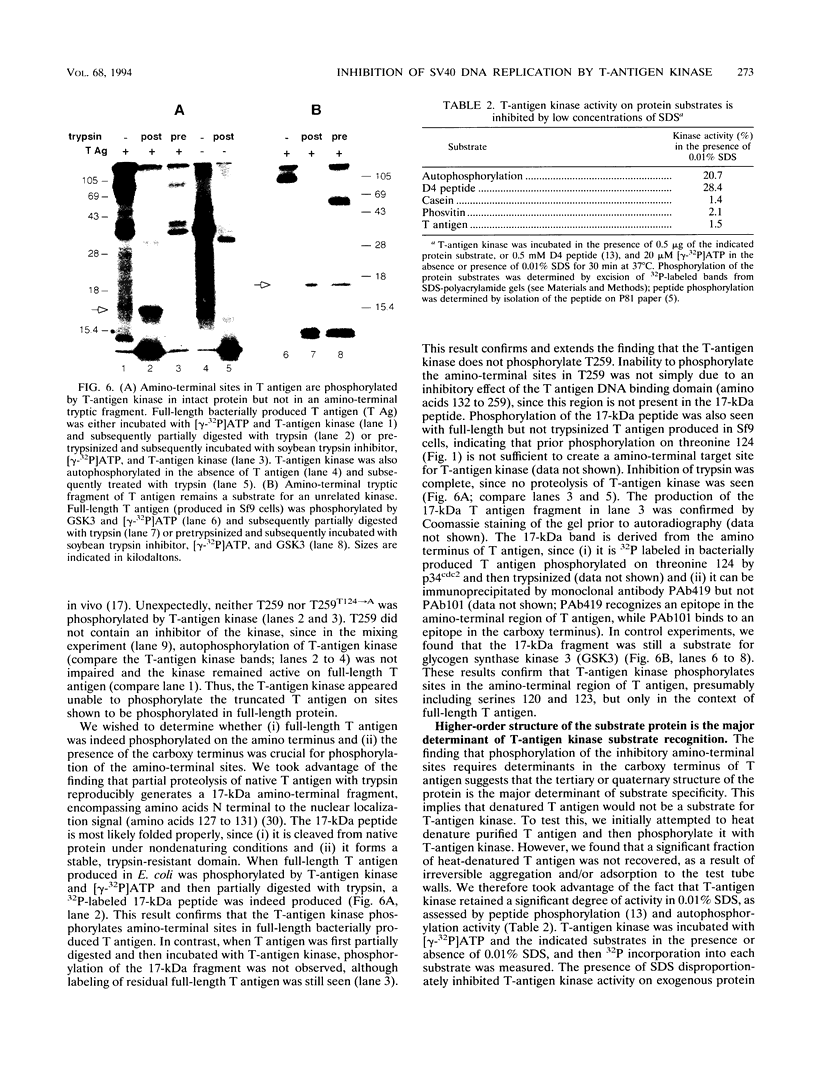
Simian virus 40 (SV40) DNA replication begins after two large T-antigen hexamers assemble on the viral minimal origin of replication and locally unwind the template DNA. The activity of T antigen in this reaction is regulated by its phosphorylation state. A form of casein kinase I purified from HeLa nuclear extracts (T-antigen kinase) phosphorylates T antigen on physiologic sites and inhibits its activity in the unwinding reaction (A. Cegielska and D. M. Virshup, Mol. Cell. Biol. 13:1202-1211, 1993). Using a series of mutant T antigens expressed by recombinant baculoviruses in Sf9 cells, we find that the origin unwinding activities of both TS677-->A and TS677,679-->A are inhibited by the T-antigen kinase, as is wild-type T antigen. In contrast, mutants TS120-->A and TS123,679-->A are resistant to inhibition by the kinase. Thus, phosphorylation of serines 120 and 123 is necessary for inhibition of T-antigen activity. Previous studies of casein kinase I substrate specificity have suggested that acidic residues or a phosphorylated amino acid amino terminal to the target residue are required to create a casein kinase I recognition site. However, we find that the T-antigen kinase can add more than 3 mol of Pi per mol to full-length bacterially produced T antigen and that it inhibits the unwinding activity of p34cdc2-activated bacterially produced T antigen. Since no prior phosphorylation is present in this bacterially produced T antigen, and no acidic residues are present immediately amino terminal to serines 120 and 123, other structural elements of T antigen must contribute to the recognition signals for T-antigen kinase. In support of this conclusion, we find that while T-antigen kinase phosphorylates amino-terminal residues in bacterially produced full-length T antigen, it cannot phosphorylate bacterially produced truncated T antigen containing amino acids 1 to 259, a 17-kDa amino-terminal tryptic fragment of T antigen, nor can it phosphorylate denatured T antigen. These findings strongly suggest that the carboxy-terminal domain of T antigen is an important modifier of the recognition signals for phosphorylation of the critical amino-terminal sites by the T-antigen kinase. This conclusion is consistent with previous studies suggesting close apposition of amino- and carboxy-terminal domains of T antigen in the native protein. The three-dimensional conformation of the substrate appears to make a significant contribution to T-antigen kinase substrate specificity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostinis P., Pinna L. A., Meggio F., Marin O., Goris J., Vandenheede J. R., Merlevede W. A synthetic peptide substrate specific for casein kinase I. FEBS Lett. 1989 Dec 18;259(1):75–78. doi: 10.1016/0014-5793(89)81498-x. [DOI] [PubMed] [Google Scholar]
- Atherton-Fessler S., Parker L. L., Geahlen R. L., Piwnica-Worms H. Mechanisms of p34cdc2 regulation. Mol Cell Biol. 1993 Mar;13(3):1675–1685. doi: 10.1128/mcb.13.3.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman J. L., Anderson R. A. Casein kinase I is regulated by phosphatidylinositol 4,5-bisphosphate in native membranes. J Biol Chem. 1991 Feb 5;266(4):2508–2512. [PubMed] [Google Scholar]
- Brockman J. L., Gross S. D., Sussman M. R., Anderson R. A. Cell cycle-dependent localization of casein kinase I to mitotic spindles. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9454–9458. doi: 10.1073/pnas.89.20.9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casnellie J. E. Assay of protein kinases using peptides with basic residues for phosphocellulose binding. Methods Enzymol. 1991;200:115–120. doi: 10.1016/0076-6879(91)00133-h. [DOI] [PubMed] [Google Scholar]
- Cegielska A., Virshup D. M. Control of simian virus 40 DNA replication by the HeLa cell nuclear kinase casein kinase I. Mol Cell Biol. 1993 Feb;13(2):1202–1211. doi: 10.1128/mcb.13.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. R., Lees-Miller S. P., Tegtmeyer P., Anderson C. W. The human DNA-activated protein kinase phosphorylates simian virus 40 T antigen at amino- and carboxy-terminal sites. J Virol. 1991 Oct;65(10):5131–5140. doi: 10.1128/jvi.65.10.5131-5140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmus M. E. Phosphorylation of eukaryotic DNA-dependent RNA polymerase. Identification of calf thymus RNA polymerase subunits phosphorylated by two purified protein kinases, correlation with in vivo sites of phosphorylation in HeLa cell RNA polymerase II. J Biol Chem. 1981 Apr 10;256(7):3332–3339. [PubMed] [Google Scholar]
- Erdile L. F., Collins K. L., Russo A., Simancek P., Small D., Umbricht C., Virshup D., Cheng L., Randall S., Weinberg D. Initiation of SV40 DNA replication: mechanism and control. Cold Spring Harb Symp Quant Biol. 1991;56:303–313. doi: 10.1101/sqb.1991.056.01.037. [DOI] [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Flotow H., Graves P. R., Wang A. Q., Fiol C. J., Roeske R. W., Roach P. J. Phosphate groups as substrate determinants for casein kinase I action. J Biol Chem. 1990 Aug 25;265(24):14264–14269. [PubMed] [Google Scholar]
- Flotow H., Roach P. J. Role of acidic residues as substrate determinants for casein kinase I. J Biol Chem. 1991 Feb 25;266(6):3724–3727. [PubMed] [Google Scholar]
- Flotow H., Roach P. J. Synergistic phosphorylation of rabbit muscle glycogen synthase by cyclic AMP-dependent protein kinase and casein kinase I. Implications for hormonal regulation of glycogen synthase. J Biol Chem. 1989 Jun 5;264(16):9126–9128. [PubMed] [Google Scholar]
- Graves P. R., Haas D. W., Hagedorn C. H., DePaoli-Roach A. A., Roach P. J. Molecular cloning, expression, and characterization of a 49-kilodalton casein kinase I isoform from rat testis. J Biol Chem. 1993 Mar 25;268(9):6394–6401. [PubMed] [Google Scholar]
- Grässer F. A., Scheidtmann K. H., Tuazon P. T., Traugh J. A., Walter G. In vitro phosphorylation of SV40 large T antigen. Virology. 1988 Jul;165(1):13–22. doi: 10.1016/0042-6822(88)90653-8. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. F., Liskay R. M., Ou A. C., DeMaggio A. J., Burbee D. G., Heffron F. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science. 1991 Aug 30;253(5023):1031–1034. doi: 10.1126/science.1887218. [DOI] [PubMed] [Google Scholar]
- Höss A., Moarefi I., Scheidtmann K. H., Cisek L. J., Corden J. L., Dornreiter I., Arthur A. K., Fanning E. Altered phosphorylation pattern of simian virus 40 T antigen expressed in insect cells by using a baculovirus vector. J Virol. 1990 Oct;64(10):4799–4807. doi: 10.1128/jvi.64.10.4799-4807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey D., Brizuela L., Mohr I., Marshak D. R., Gluzman Y., Beach D. Phosphorylation of large tumour antigen by cdc2 stimulates SV40 DNA replication. Nature. 1989 Oct 12;341(6242):503–507. doi: 10.1038/341503a0. [DOI] [PubMed] [Google Scholar]
- McVey D., Strauss M., Gluzman Y. Properties of the DNA-binding domain of the simian virus 40 large T antigen. Mol Cell Biol. 1989 Dec;9(12):5525–5536. doi: 10.1128/mcb.9.12.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne D. M., Palmer R. H., Campbell D. G., Meek D. W. Phosphorylation of the p53 tumour-suppressor protein at three N-terminal sites by a novel casein kinase I-like enzyme. Oncogene. 1992 Jul;7(7):1361–1369. [PubMed] [Google Scholar]
- Mohr I. J., Gluzman Y., Fairman M. P., Strauss M., McVey D., Stillman B., Gerard R. D. Production of simian virus 40 large tumor antigen in bacteria: altered DNA-binding specificity and dna-replication activity of underphosphorylated large tumor antigen. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6479–6483. doi: 10.1073/pnas.86.17.6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowles J., Slaughter C., Moomaw C., Hsu J., Cobb M. H. Purification of casein kinase I and isolation of cDNAs encoding multiple casein kinase I-like enzymes. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9548–9552. doi: 10.1073/pnas.88.21.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Buck M., Schneider J., Kalderon D., Fanning E., Smith A. E. Biochemical characterization of phosphorylation site mutants of simian virus 40 large T antigen: evidence for interaction between amino- and carboxy-terminal domains. J Virol. 1991 Mar;65(3):1479–1490. doi: 10.1128/jvi.65.3.1479-1490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H. Phosphorylation of simian virus 40 large T antigen: cytoplasmic and nuclear phophorylation sites differ in their metabolic stability. Virology. 1986 Apr 15;150(1):85–95. [PubMed] [Google Scholar]
- Scheidtmann K. H., Schickedanz J., Walter G., Lanford R. E., Butel J. S. Differential phosphorylation of cytoplasmic and nuclear variants of simian virus 40 large T antigen encoded by simian virus 40-adenovirus 7 hybrid viruses. J Virol. 1984 May;50(2):636–640. doi: 10.1128/jvi.50.2.636-640.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Virshup D. M., Kelly T. J. Protein phosphatase 2A dephosphorylates simian virus 40 large T antigen specifically at residues involved in regulation of DNA-binding activity. J Virol. 1991 Apr;65(4):2098–2101. doi: 10.1128/jvi.65.4.2098-2101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Fanning E. Mutations in the phosphorylation sites of simian virus 40 (SV40) T antigen alter its origin DNA-binding specificity for sites I or II and affect SV40 DNA replication activity. J Virol. 1988 May;62(5):1598–1605. doi: 10.1128/jvi.62.5.1598-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer M., Weil R., Frank G., Zuber H. Amino acid sequence analysis of fragments generated by partial proteolysis from large simian virus 40 tumor antigen. J Biol Chem. 1980 Jun 25;255(12):5627–5634. [PubMed] [Google Scholar]
- Strauss M., Argani P., Mohr I. J., Gluzman Y. Studies on the origin-specific DNA-binding domain of simian virus 40 large T antigen. J Virol. 1987 Oct;61(10):3326–3330. doi: 10.1128/jvi.61.10.3326-3330.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon P. T., Traugh J. A. Casein kinase I and II--multipotential serine protein kinases: structure, function, and regulation. Adv Second Messenger Phosphoprotein Res. 1991;23:123–164. [PubMed] [Google Scholar]
- Umphress J. L., Tuazon P. T., Chen C. J., Traugh J. A. Determinants on simian virus 40 large T antigen are important for recognition and phosphorylation by casein kinase I. Eur J Biochem. 1992 Jan 15;203(1-2):239–243. doi: 10.1111/j.1432-1033.1992.tb19852.x. [DOI] [PubMed] [Google Scholar]
- Virshup D. M., Kauffman M. G., Kelly T. J. Activation of SV40 DNA replication in vitro by cellular protein phosphatase 2A. EMBO J. 1989 Dec 1;8(12):3891–3898. doi: 10.1002/j.1460-2075.1989.tb08568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M., Kelly T. J. Purification of replication protein C, a cellular protein involved in the initial stages of simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1989 May;86(10):3584–3588. doi: 10.1073/pnas.86.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M., Russo A. A., Kelly T. J. Mechanism of activation of simian virus 40 DNA replication by protein phosphatase 2A. Mol Cell Biol. 1992 Nov;12(11):4883–4895. doi: 10.1128/mcb.12.11.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]