Abstract
Triabin, a 142-residue protein from the saliva of the blood-sucking triatomine bug Triatoma pallidipennis, is a potent and selective thrombin inhibitor. Its stoichiometric complex with bovine α-thrombin was crystallized, and its crystal structure was solved by Patterson search methods and refined at 2.6-Å resolution to an R value of 0.184. The analysis revealed that triabin is a compact one-domain molecule essentially consisting of an eight-stranded β-barrel. The eight strands A to H are arranged in the order A-C-B-D-E-F-G-H, with the first four strands exhibiting a hitherto unobserved up-up-down-down topology. Except for the B-C inversion, the triabin fold exhibits the regular up-and-down topology of lipocalins. In contrast to the typical ligand-binding lipocalins, however, the triabin barrel encloses a hydrophobic core intersected by a unique salt-bridge cluster. Triabin interacts with thrombin exclusively via its fibrinogen-recognition exosite. Surprisingly, most of the interface interactions are hydrophobic. A prominent exception represents thrombin’s Arg-77A side chain, which extends into a hydrophobic triabin pocket forming partially buried salt bridges with Glu-128 and Asp-135 of the inhibitor. The fully accessible active site of thrombin in this complex is in agreement with its retained hydrolytic activity toward small chromogenic substrates. Impairment of thrombin’s fibrinogen converting activity or of its thrombomodulin-mediated protein C activation capacity upon triabin binding is explained by usage of overlapping interaction sites of fibrinogen, thrombomodulin, and triabin on thrombin. These data demonstrate that triabin inhibits thrombin via a novel and unique mechanism that might be of interest in the context of potential therapeutic applications.
Thrombin (E.C. 3.4.21.5), the ultimate serine proteinase formed via activation of the blood coagulation cascade, is the principal promoter of blood clotting. It activates the thrombin receptor resulting in platelet stimulation and aggregation, and converts fibrinogen to fibrin, which subsequently polymerizes to form the solid fibrillar matrix of the blood clot. Thrombin also plays an important role in the counterbalance of blood clotting via the protein C pathway and is known to be a potent mitogen for vascular smooth muscle cells (1). Essential for the high specificity of thrombin are its prominent active-site cleft as well as two anion-binding exosites located far from the catalytic center (2–4). The active-site cleft is narrowed to a deep canyon, which limits access of macromolecular substrates and inhibitors to the catalytic residues (2, 5, 6). The two exosites are surface patches mainly composed of positively charged residues. Exosite II serves as an anchoring region for heparin to clamp serpins and α-thrombin together, thus accelerating complex formation (7). Exosite I, also termed fibrinogen-recognition exosite (FRE), can be subdivided into a more “proximal” region consisting of the “eastern” part of the active-site cleft, and a more “distal” one where the active-site cleft passes over to the adjacent convex surface of thrombin (to the “east” in Fig. 1). The FRE is utilized by thrombin for selective binding of protein inhibitors and cofactors and is implicated in recognition and cleavage of specific macromolecular substrates (5, 9).
Figure 1.
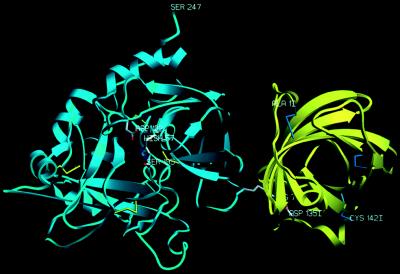
Ribbon plot of the complex formed between bovine α-thrombin (blue) and triabin (yellow). The proteinase is shown in its “standard” orientation—i.e., with the active-site cleft facing the viewer and binding peptide substrates running from left to right. Side chains of the catalytic triad residues of thrombin are explicitly shown, as well as the important interface residues Arg-77A (thrombin) and Asp-135I (triabin). The figure was made with setor (8).
Normally, the catalytic activity of thrombin in vivo is controlled by a few endogenous serum inhibitors such as α2-macroglobulin and the serpins antithrombin III, heparin cofactor II, and proteinase nexin I. Thrombin activity at the wrong place and on the wrong time can cause life-threatening clinical emergencies such as myocardial infarction, stroke, or pulmonary embolism. Therefore, thrombin is considered a prime target for the prevention of pathological clot formation (10). Inhibitors produced by various haematophageous animals have therefore evoked the interest of biochemists, pharmacologists, and clinicians alike for many years. To avoid blood clotting during feeding and digestion, the saliva of these parasites contains proteinase inhibitors directed toward several blood coagulation factors of their prey, most notably toward thrombin (11). Of the several thrombin inhibitors from these blood-sucking animals known to date, the structures of the thrombin complexes with hirudin (12, 13), rhodniin (14), and ornithodorin (15) have been studied by x-ray crystallography. A common feature of these nonhomologous inhibitors is their two-domain structure, with the N-terminal domain binding directly to or adjacent to thrombin’s active site, and the C-terminal domain interacting with its FRE. Consequently, the corresponding thrombin complexes lack any proteolytic activity, both toward small chromogenic substrates as well as toward macromolecular protein substrates.
In contrast, triabin, an unrelated 142-amino acid residue polypeptide isolated from the triatomine bug Triatoma pallidipennis (Hemiptera, family Reduviidae, subfamily Triatominae), reduces thrombin’s hydrolytic activity toward chromogenic substrates only slightly. It forms a noncovalent 1:1 complex with thrombin, inhibits thrombin-induced platelet aggregation, and prolongs the thrombin clotting time. With fibrinogen as a substrate, the dissociation constant of the thrombin–triabin complex was determined to about 3 pM. However, a small but definite constant activity level remains after equimolar complex formation (refs. 16 and 17; and C.N.-J. and W.-D.S., unpublished data). Triabin was furthermore shown to inhibit the thrombomodulin-mediated activation of protein C by thrombin. These observations led to the assumption that triabin binds to thrombin at a surface site(s) located more distantly from the active site, most probably to its FRE or to both anion-binding exosites and provoked a detailed structural investigation. In the following, we show crystallographically that triabin indeed interacts with the FRE of its cognate thrombin.
MATERIALS AND METHODS
Purification and Crystallization.
Recombinant triabin was overexpressed in insect cells using a baculovirus-driven expression system and purified as described (18). Bovine thrombin was purified as reported elsewhere (19). After incubating equimolar amounts of both proteins for 30 min on ice, the complex was subjected to anion-exchange chromatography on a 7.5 mm × 7.5 cm DEAE-5PW column (TosoHaas, Stuttgart, Germany). Fractions containing the equimolar complex were dialyzed against 10 mM Na acetate, 25 mM NaCl (pH 5.5), concentrated in Centricon 30 tubes (Amicon), and used for sitting-drop vapor-diffusion crystallization. Good crystals grew within a week at 20°C in drops containing 50 mM Na acetate (pH 4.6), 100 mM (NH4)2SO4, 0.01% NaN3, and 8% PEG 4000 (start concentration of the complex, ≈2.5 mg/ml), concentrated against a reservoir solution containing 16% PEG 4000. Crystals used for data collection reached maximal dimensions of 0.7 mm × 0.15 mm × 0.1 mm.
Data Collection and Processing.
Diffraction data to 2.6 Å were collected at 7°C on a 300 mm MAR image plate system (MAR Research, Hamburg) using monochromatized CuKα radiation produced by a rotating anode generator (Table 1). These data were evaluated with the mosflm package (20) and merged and scaled using programs of the CCP4 program suite (21). The crystals belong to the orthorhombic space group P212121, contain one complex molecule per asymmetric unit, and diffract to beyond 2.6 Å.
Table 1.
Summary of crystallographic data
| Measurement | Value |
|---|---|
| Space group | P212121 |
| Cell constants | a = 44.84 Å, b = 67.23 Å, c = 183.49 Å |
| Limiting resolution, Å | 2.60 |
| Significant measurements | 79,890 |
| Rmerge,* % | 9.9 |
| Independent reflections | 16,392 |
| Completeness (∞–2.60 Å), % | 93.2 |
| Outermost shell (2.67–2.60 Å), % | 81.0 |
| Nonhydrogen protein atoms | 4,304 |
| Solvent molecules | 172 |
| Reflections used for refinement | 14,627 |
| Resolution range, Å | 10.0–2.6 |
| R value,† % | 18.4 |
| Rfree,‡ % | 27.5 |
| rms SD | |
| Bond lengths, Å | 0.008 |
| Main chain bond angles, ° | 1.49 |
| Side chain bond angles, ° | 25.92 |
Rmerge: ΣĥΣî|I(hî) − 〈I(h)〉|)/ΣĥΣî I(hî), where I(hi) is the intensity of the hth reflection as determined by the ith measurement, and 〈I(h)〉 is the average value.
R value: Σ(|Fobs| − |Fcalc|)/Σ|Fobs|, where |Fobs| is the observed and |Fcalc| is the calculated structure factor amplitude of reflection hkl.
Rfree: calculated for a randomly selected test set comprising 5% of all reflections, that were excluded for a refinement performed after heating the model to 3,000 K.
Structure Determination.
Searches for the orientation and position of the thrombin molecule in the crystals were performed with the program suite amore (22) using diffraction data from 9.0 to 3.5 Å and the coordinates of bovine α-thrombin as observed in its complex with rhodniin (14). In the search model, residues Thr-1U to Asp-1A, and Leu-14L to Arg-15 of the A chain and most of the residues forming the autolysis loop (Glu-146 to Glu-149E) were excluded. Rotational search yielded two possible solutions both with correlation factors of 0.211; only one of them gave rise to a favorable translation solution. After fitting, this unique solution exhibited a correlation value of 0.555 and an R factor of 0.382 (values for the next highest peak: c = 0.131, r = 0.525). A 3.0-Å Fourier map calculated after appropriate positioning of the thrombin molecule in the crystal cell allowed building of the autolysis loop and addition of the omitted parts of the thrombin A chain. In addition, some stretches of continuous electron density were visible near the fibrinogen-recognition exosite of thrombin accounting for three polyalanine strands of triabin, which were modeled into this density on a Silicon Graphics workstation using main (23). The complex was crystallographically refined with x-plor (24) using the target parameters of Engh and Huber (25). This procedure was cyclically repeated a few times, until several side chains were identified so that the correct amino acid residues could be built in. After the whole inhibitor polypeptide chain [except segments Gly-46I to Lys-48I and Asn-74I to Lys-75I; the suffix “I” serves to distinguish triabin from thrombin residues, with residues of the latter identified by chymotrypsinogen numbering deduced from topological equivalencies (3, 4)], was fitted to the density map, water molecules were added at stereochemically reasonable positions at 1σ and 2σ peaks in the (2Fobs − Fcalc) density and (Fobs − Fcalc) difference density maps. Finally, individual but highly restrained B values were refined.
The final model comprises residues Phe-1M to Arg-15 of the A chain, all 259 residues of the B chain, segments Ala-1I to Ser-45I, Gly-49I to Asp-73I, and Asn-76I to Cys-142I of triabin, and 172 water molecules. Two discrepancies with the previously reported protein sequence (Arg-123I instead of Asp and Phe-127I instead of Leu) were found and verified by sequencing both strands of the gene. Weak electron density near Asn-60G Nɛ2 and Asn-22I Nɛ2, possibly accounting for the carbohydrate moieties of the single α-thrombin sugar chain and of the inhibitor, respectively, was left uninterpreted. The R factor of this final model is 0.184 for reflections between 10.0 and 2.6 Å (with a free R factor of 0.275). All main chain dihedral angles of nonglycine residues fall into “allowed” regions of the Ramachandran plot, with 85.6% of them in the most favored conformations (26). A summary of the crystal and refinement data is presented in Table 1. The atomic coordinates and structure factors will be deposited in the Brookhaven Protein Data Bank (reference number 1avg).
Structural Comparisons.
A search for topologically equivalent proteins deposited with the Protein Data Bank was performed with the program gdf3d (27), with the maximal distance of matching Cα atoms set to 1.8 Å for polypeptide stretches containing at least six residues. Comparisons of three-dimensional structures were performed by least-squares methods with frodo (28), using the coordinate sets from the Protein Data Bank 1bbp (for bilin binding protein; ref. 29) and 1brp (for human retinol binding protein; ref. 30).
RESULTS
Overall Structure The crystals contain a single thrombin–triabin complex per asymmetric unit.
Both molecules are in contact with one another (see below), but also with molecules of symmetry-related complexes. Each thrombin molecule interacts through its A chain C-terminal segment (residues Ile-14K to Arg-15) with nonprimed subsites of a neighboring thrombin molecule in a substrate/product-like manner, reminiscent of the factor Xa crystals (31)—i.e., the Arg-15 side chain inserts into the S1 pocket, Glu-14L, like typical P3-residues, binds through two hydrogen bonds to Gly-216, and the Ile-14K side chain, like that of other L-configured P4-residues, slots into the aryl (S3/S4) binding site (4, 32). In the thrombin–triabin crystals, this inter-thrombin interaction gives rise to the formation of extended chains of assembled thrombin molecules making the crystal “scaffold” (data not shown).
Each triabin molecule is in direct contact with two thrombin molecules through two very different surface patches. The first of these intermolecular contacts (described in detail below) comprises 90 nonhydrogen atom–atom contacts below 4.0 Å, which are made upon removal of 940-Å2 molecular surfaces of each molecule from bulk water contact during association. The second interaction surface, comprising 61 such contacts gives rise to the burial of 445-Å2 surfaces of each molecule and includes six loop residues of the inhibitor. Due to the larger number of contacts and the superior shape and charge complementarity within the first intermolecular interface, and because of the correspondence with our previous experimental data (16, 17), we are confident that the “solution” complex corresponds to the binary complex shown as a ribbon diagram in Fig. 1.
In the complex, the thrombin component differs only slightly from other reported thrombin structures (4). Therefore, complex formation with triabin does not result in significant gross conformational changes in the thrombin component, in accord with the minor (30%) decrease in catalytic activity of thrombin upon complex formation (16).
Triabin Structure Triabin is a single domain molecule of approximate dimensions 32 Å × 30 Å × 25 Å.
Its main structural feature is an eight-stranded β-sheet, which is smoothly rolled and annealed at both edges forming a slightly flattened and conical β-barrel with the overall appearance of a calyx (Fig. 2). The sheet is characterized by a shear number of 12 (see Fig. 3). The first (A) and the last strand (H) are aligned with each other under an angle of about 30°, hydrogen-bonded between Tyr-26I (strand A) and Glu-128I (strand H). This contrasts with the extended hydrogen-bonding network formed between all other pairs of neighboring strands within the β-sheet, which are cross-linked through 5–10 hydrogen bonds (see Fig. 3 for a schematic representation of triabin’s secondary structure). The first four N-terminal strands (labeled A to D) are adjacently arranged in the order A, C, B, D with an unusual up-up-down-down or 2x, -1, 2x topology. They are connected by relatively long loops, with the B/C loop (loops are identified by the strands flanking them) being partially disordered in our crystals. The A/B and the C/D loops project into the wider “back” opening of the conical barrel, acting together with the side chains of loop residues His-29I, Tyr-58I, Lys-60I, and Phe-61I like a lid of the calyx (see Fig. 2).
Figure 2.
Stereoview of the triabin ribbon. The eight strands A to H forming the β- barrel are shown as yellow arrows, the N- and the C-terminal surface helices are shown as green helical ribbons, and the connecting loops are shown as green ropes. A few aromatic side chains, as well as the polar residues involved in the internal salt-bridge cluster, are shown with all nonhydrogen atoms. The view is, similar as in Fig. 1, approximately along the barrel axis of triabin—i.e., through the more narrow barrel opening (front) toward the wider opening of the calyx (back). The figure was made with setor (8).
Figure 3.
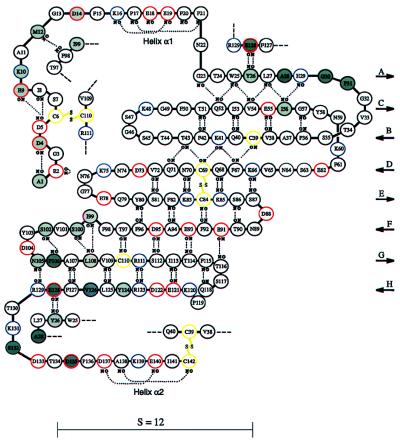
Schematic representation of the polypeptide chain arrangement of triabin. Acidic, basic, and cysteine residues are emphasized with red, blue, and yellow circles, respectively. Disulfide bridges are shown explicitly, and inter-main chain hydrogen bonds are represented as dotted lines. Residues involved in interactions with thrombin are shadowed in dark gray if in contact with Tyr-76 and/or Arg-77A, and in light gray otherwise. The shear number S equals the residue offset upon surrounding the barrel.
The five C-terminal strands D to H are arranged in a regular W-like up-and-down manner; the last four of them (E to H) are connected by short, well-defined β-hairpin loops. The barrel interior is densely packed with hydrophobic (mainly aromatic) side chains donated from all eight strands (Fig. 2). It also includes disulfide bridge Cys-69I/Cys-84I connecting the spatially adjacent strands D and E. The interior of the barrel is crossed by a polar cluster, which is fully embedded in the hydrophobic core and clamps opposite barrel strands tightly together. Central to this cluster is the Glu-55I (strand C) carboxylate, which asymmetrically opposes the guanidinium group of Arg-111I (strand G), forming a forked 1N—2O hydrogen bond. The Glu-55I carboxylate group is further flanked by the terminal carboxamide group of Gln-40I (strand B) and by the imidazole ring of His-29I (strand A), forming Nɛ—Oɛ hydrogen bonds to either side (Fig. 2).
The N-terminal segment (Ala-1I to Phe-21I) crosses the barrel side on top of β-strands F, G, and H and is covalently linked to strand G via disulfide bridge Cys-6I/Cys-110I. It terminates in a short mixed 310-α-helix (h1), which acts like a lid at the “front” barrel opening (Fig. 2). Also the C-terminal peptide of triabin (Thr-130I to Cys-142I) is located on the outer barrel surface and covers the external faces of the N-terminal strands A, C, and B. The short C-terminal helix h2 is covalently clamped to strand B through disulfide bridge Cys-39I/Cys-142I (Fig. 2). Thus, all six cysteine residues of triabin are disulfide-paired according to the connectivity pattern abccab linking together sequentially distant parts of the polypeptide chain.
Interaction Interface Thrombin contacts the triabin molecule exclusively via its FRE (Fig.
1). Both contacting surfaces are slightly convex and rectangular in shape, surrounded by side pockets filled with a number of (mainly ordered) solvent molecules, eight of which (interpreted as water molecules) directly cross-connect polar groups from both proteins. A total of 19 triabin residues (shadowed in Fig. 3) and 13 thrombin residues participate in direct contacts, most of which are made between side chains. On the thrombin side, four different polypeptide segments (Thr-74 to Met-84, Lys-36 to Gln-38, Leu-65, and Lys-109/Arg-110) provide contacting residues. These residues comprise the “distal” part of the FRE, spanned between Arg-75 and Lys-78 (southern front and back) and Lys-36 and Arg-110 (northern front and back, see Fig. 4).
Figure 4.
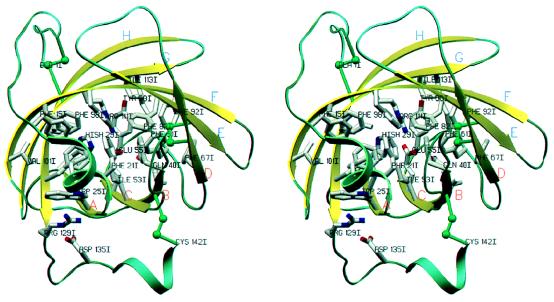
Closed-up stereo-view of the interaction interface between bovine thrombin and triabin. The contacting segments of thrombin (blue) and triabin (yellow) are shown as α-carbon traces, and only the more important side chains are given with all atoms. Water molecules are omitted for the sake of simplicity. Orientation is similar to that seen in Figs. 1 and 2. The figure was made with setor (8).
The larger northern region of this interface is essentially made by hydrophobic side chains such as Ile-99I, Leu-108I, Val-126I, and Phe-106I of triabin, and Leu-65 and Met-84 of thrombin, whereas toward its periphery also a few polar residues, such as Glu-9I and the distal ammonium groups of Lys-36 and Lys-109, form intermolecular contacts. This northern interface region is separated by a water-filled notch and the Tyr-76 side chain from a smaller and much more polar southern region, which is dominated by an intermolecular ion pair/hydrogen bond cluster. Here, the almost extended thrombin side chain of Arg-77A fits into a mostly hydrophobic triabin pocket, where its terminal guanidinium group opposes the carboxylate group of Asp-135I forming symmetrical charged 2N—2O hydrogen bonds, which are fully shielded from the bulk solvent and thus seem to be quite strong. Aside of this Arg-77A guanidinium group, the carboxylate of Glu-128I is suitably placed to make an additional charged Nɛ—Oɛ hydrogen bond, which, in contrast to the Arg-77A/Asp-135I bonds, is flanked by ordered solvent molecules and thus is presumably not of comparable strength. Placed on top of the proximal part of the Arg-77A side chain and aside the carboxylate group of Glu-128I, the Tyr-76 side chain of thrombin is directed toward a water-filled pocket extending from the bulk water into the interface. With its surface-directed side, this Tyr-76 phenolic ring packs further against the hydrophobic chains of triabin residues Phe-106I and Val-126I (see Fig. 4). The side chains of Tyr-76 and Arg-77A together account for almost half of all atom–atom contacts below 4.0 Å made by the thrombin component.
Structural Similarities to Lipocalins The eight-stranded β-barrel of triabin is reminiscent of the lipocalins, a family of medium-sized proteins comprising an eight-stranded calyx-shaped β-barrel, which in most cases encloses an internal cavity suitable for binding small hydrophobic molecules (see refs.
33 and 34). Typical representatives of this widespread family are the bilin binding protein (BBP) from the insect Pieris brassicae (29), and the mammalian retinol binding proteins (RBPs; refs. 30 and 35). In contrast to triabin, however, the eight β-strands of these “classical” lipocalins are arranged in sequential order, with adjacent strands aligned antiparallel to one another.
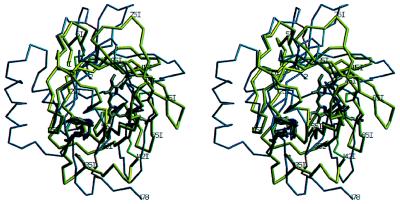
A systematic computer search conducted against all protein structures available in the Protein Data Bank at the end of 1996 (36) indeed revealed a striking similarity between triabin and some lipocalins, but failed to suggest structural relations to any other protein. A superposition with BBP and RBP showed that more than half of all Cα atoms of triabin occupy positions within 2.5-Å distance of equivalent Cα atoms of the lipocalins, with rms deviations of 1.5 Å. All eight triabin β-strands (neglecting strand directions) and most of the N-terminal segment superimpose astonishingly well. Strands B and C, although interchanged, also have very similar curvatures, as emphasized by the fact that eleven Cα atoms of these strands can be superimposed (see Fig. 5).
Figure 5.
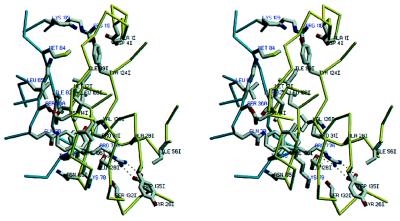
Stereoview of the Cα structures of triabin (yellow) and of the bilin binding protein (blue) after optimal least-squares fit of the eight strands. The residue numbering is that for triabin. The bound biliverdin pigment (gray) is presented to indicate the cavity in the ligand binding lipocalins. The side chains of residues Trp-25I and Arg-129I and of the triabin specific residue Asp-135I are given in full length. The figure made with setor (8).
The similarity between triabin and the classical lipocalins extends to single residues such as the Asp-14I/Phe-15I pair positioned at the entrance to helix h1, and the adjacent Phe-20I, with both Phe side chains participating in the “front” barrel lid (see Figs. 2 and 6). Strand A, as in all other crystallographically studied lipocalins, starts with a strictly conserved Gly-XAA-Trp motif, followed by an aromatic residue. This Gly-23I exhibits a high energy main chain conformation (Θ = +67°, Ψ = −151°) usually confined to Gly residues, and the indolyl side chain of Trp-25I is packed in the interior of the barrel. Similar to almost all lipocalins, the Arg-129I side chain of triabin at the C-terminal end of strand H packs against this indol ring, shielding it from the bulk water. The last four barrel strands E to H of triabin share several common sequence motifs with the lipocalins (see Fig. 6).
Figure 6.
Structure-based alignment of the amino acid sequences of triabin and of the lipocalins of known three-dimensional structure: retinol binding protein (RETB_HUMAN; refs. 30 and 35), odorant binding protein (OBP_BOVIN; ref. 37), major urinary protein (MUP1_MOUSE; ref. 38), insecticyanin (ICYA_MANSE; ref. 39), retinoic acid binding protein (ERBP_RAT; ref. 40), and bilin binding protein (BBP_PIER; ref. 29). The three major structurally conserved regions (SCRs 1–3) are indicated. Numbers refer to the triabin sequence. Topologically equivalent residues between triabin and retinol binding protein are indicated by asterisks. Note that they correspond to the “reversed” sequence for residues 29I to 64I. The aligned sequences were formatted using the program alscript (41).
The loops connecting the β-strands show greater differences. The large A/B loop is most variable also among the classical lipocalins, where it forms almost half of the wall surrounding the entrance to their internal cavities. The G/H loop of triabin folds over the “back” opening of the barrel, forming together with side chains His-29I (A/B loop), and Tyr-58I, Lys-60I, and Phe-61I (C/D loop), a lid covering triabin’s internal core. The equivalent barrel side in the ligand-binding lipocalins is open and represents the entrance to the internal binding cavities (see Fig. 5). Also the C-terminal segment of triabin deviates strongly from the standard lipocalin fold. Lack of a 3.5 turn α-helix, as well as of an additional β-strand present in most lipocalins, essentially accounts for the lower molecular weight of triabin (142 residues) compared with the classical lipocalins (from 160 to more than 190 residues). In spite of the considerable topological similarity, the sequence identity between triabin and lipocalins such as BBP or RBP is rather low. As shown in Fig. 6, about 20% of all amino acid residues occupying topologically equivalent strand positions are identical in triabin–BBP or triabin–RBP pairs. This value is only slightly lower than the residue identity between BBP and RBP, but still too low to be statistically significant on its own.
DISCUSSION
We have solved the crystal structure of the complex made between bovine thrombin and the protein inhibitor triabin, and have identified the solution complex. The most important results of this analysis are that (i) the triabin molecule is structurally quite closely related to the lipocalins, and (ii) it docks to the “distal” part of the fibrinogen-recognition exosite of the thrombin molecule.
Triabin’s topological similarity with the lipocalins is indeed considerable and supports the idea that it might have evolved from a common lipocalin ancestor by divergent evolution. This structural similarity is not restricted to the overall appearance of the barrel, but extends also to details such as the strictly conserved Gly-23I, the preserved Trp-25I/Arg-129I pair, a number of side chains of β-strands E to H, or the connecting loops positioned at the front exit of the barrel (Figs. 1, 2, and 5). The closest similarities (Figs. 5 and 6) are observed for the chain part comprising helix h1 and strand A, strands F and G, and strand H together with the adjacent loop. These regions have previously been found to be particularly preserved in lipocalins, and have therefore been grouped into three “structurally conserved regions” (33).
Triabin notably differs from the ligand-binding lipocalins (for which three-dimensional information is available) by the topological interchange of strands B and C, resulting in a different organization of the adjacent A/B and C/D loops, the lack of an internal cavity, and a much shorter C-terminal segment. Such a strand interchange, accompanied by the filling out of the internal cavity and shortening and remodeling of the long C-terminal segment during evolution, is conceivable.
Pallidipin, a potent inhibitor of the collagen-induced platelet aggregation which we have identified in the saliva of T. pallidipennis (42), and a similar inhibitor more recently isolated from the assassin bug Rhodnius prolixus (D. E. Champagne, J. F. Andersen, and J. M. C. Ribeiro, personal communication) display a fairly high degree (≈45%) of sequence similarity with triabin, probably reflecting a very similar peptide fold and internal organization. Interestingly, these aggregation inhibitors have an extended C terminus, similar to the other lipocalins. Thus, the short C terminus seems to be a special feature of triabin. In lipocalins such as BBP the long C-terminal helix mainly covers the outer face of the G–H sheet, whereas this G–H surface of triabin is part of the site of interaction with thrombin (Fig. 5). The equivalent barrel surface (which is placed roughly opposite to the cavity entrance) has been suggested to represent the receptor binding site and/or macromolecular complexation site in some other lipocalins (33). Thus, triabin might represent the first member of a new lipocalin subclass, for which this protein recognition site is known in atomic detail. Whether the counterparts of this thrombin-recognition surface in other lipocalins are, at least partially, involved in receptor binding or in other macromolecular interactions, is an attractive hypothesis that could be tested by further structural investigations and/or site-directed mutagenesis.
The second important finding of this structure analysis is that thrombin binds to the triabin molecule exclusively via the “distal” convex part of its basically charged FRE. In this complex, thrombin utilizes almost the same surface regions as it does in the interactions with the second domains of the insect derived thrombin inhibitors rhodniin (14) and ornithodorin (15), which exhibit, however, quite different folds belonging to the Kazal- and the Kunitz inhibitor family, respectively (32). Due to its much larger size (142 residues, compared with 48 and 58), triabin binds across a surface almost doubling that of the other two inhibitors, allowing some additional interactions. Surprisingly, most of the contacts in thrombin–triabin are made through hydrophobic side chains. The only important salt-bridge interactions are formed between Arg-77A and triabin’s Asp-135I and Glu-128I. Besides this ion pair cluster, the negatively and positively charged residues of triabin and thrombin, respectively, will assist in binding through their opposite electrostatic fields. Due to the predominance of negatively over positively charged residues in triabin (12 Glu and 11 Asp residues oppose 12 Lys and 3 Arg residues, see Fig. 3), a larger number of direct ion pairs had been anticipated, in particular participation of triabin residues harbored in segments with successive acidic residues. The Glu and Asp residues flanking Asp-135I (Fig. 3) are directed away from the thrombin surface, however. This low ratio of direct ion pairs is reminiscent of the FRE interactions with the C-terminal tail/domains of hirudin (12, 13), rhodniin (14), and ornithodorin (15), where likewise the majority of the oppositely charged residues are found to interact via the overall electrostatic potentials.
In contrast to these other three thrombin-specific protein inhibitors, triabin occupies exclusively the FRE of thrombin, thus leaving the active site of the liganded enzyme free to act on small peptidic molecules. It has been long known from experiments with the native and with proteolytically cleaved thrombin species that thrombin’s FRE and its integrity are essential for a number of thrombin functions such as fibrinogen clotting, thrombomodulin dependent protein C cleavage, and thrombin receptor (platelet) activation (refs. 2 and 4, and references therein). More recent mutation experiments seem to indicate that the FRE residues Arg-73, Tyr-76, Asn-78, Lys-81, Lys-109, and Arg-110 are particularly critical for fibrinogen recognition and cleavage, whereas Gln-38, Arg-75, and Tyr-76 mainly participate in thrombin–thrombomodulin interactions (43, 44). The latter interactions would be in agreement with the crystal structure of a thrombin complex formed with a loop segment of the epidermal growth factor 5 (EGF-5) domain of thrombomodulin (45). According to crystal structures of thrombin in complex with receptor peptides (46), the cleavage segment of the thrombin receptor seems to insert into the FRE at least up to Tyr-76, Ile-82, and Lys-36. All of these interaction sites might not be identical, but clearly overlap and include residues, which are also in direct contact with triabin. Thus, triabin does compete for FRE sites with several macromolecules, explaining its measured inhibitory effect on fibrinogen cleavage and platelet stimulation. All of these contacting residues of the FRE region seem to be conserved in all mammalian thrombin species (47), so that similar interactions are anticipated for triabin and for these macromolecular substrates/effectors of thrombin.
The faint, but measurable fibrinogen clotting activity of the triabin-thrombin complex in the presence of excess triabin might indicate that fibrinogen might approach and bind to thrombin not in a rigid body encounter, but with some degree of molecular flexibility. This fibrinogen cleavage “leakage” of the thrombin–triabin complex is a particularly interesting feature for clinical applications, because it might allow a less stringent adjustment. Possibly, the interference of triabin with some other substrates/inhibitors, which occupy only a small contact region around thrombin’s active site upon binding, is much weaker or even negligible. Thus, triabin might offer advantages over other active-site directed antithrombotics.
Acknowledgments
We thank S. Kaloudin and D. Grosse for bovine thrombin purification, Dr. Mann for the N-terminal sequencing of the crystallized material, and Dr. Gomis-Rüth and Dr. Medrano for computational help. The financial support of the Sonderforschungsbereich (SFB 469), European Union Biomed Project BMH4-CT96-0937, and Fonds der Chemischen Industrie are greatly acknowledged.
ABBREVIATIONS
- FRE
fibrinogen recognition exosite
- BBP
bilin binding protein
- RBP
retinol binding protein
Footnotes
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, Biology Department, Brookhaven National Laboratory, Upton, NY 11973 (reference 1avg).
References
- 1.Davie E W, Fujikawa K, Kisiel W. Biochemistry. 1991;30:10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 2.Stubbs M T, Bode W. Trends Biochem Sci. 1995;20:23–28. doi: 10.1016/s0968-0004(00)88945-8. [DOI] [PubMed] [Google Scholar]
- 3.Bode W, Mayr I, Baumann U, Huber R, Stone S R, Hofsteenge J. EMBO J. 1989;8:3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode W, Turk D, Karshikov A. Protein Sci. 1992;1:426–471. doi: 10.1002/pro.5560010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stubbs M T, Oschkinat H, Mayr I, Huber R, Angliker H, Stone S R, Bode W. Eur J Biochem. 1992;206:187–192. doi: 10.1111/j.1432-1033.1992.tb16916.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin P D, Robertson W, Turk D, Huber R, Bode W, Edwards B F P. J Biol Chem. 1992;267:7911–7920. [PubMed] [Google Scholar]
- 7.Olson S T, Bjørk I. In: Thrombin. Berliner L J, editor. New York: Plenum; 1992. pp. 159–217. [Google Scholar]
- 8.Evans S V. J Mol Graphics. 1993;11:134–138. doi: 10.1016/0263-7855(93)87009-t. [DOI] [PubMed] [Google Scholar]
- 9.Stubbs M T, Bode W. Perspect Drug Discovery Design. 1993;1:431–452. [Google Scholar]
- 10.Fenton J W, II, Ofosu F A, Mooon D G, Maraganore J M. Blood Coagulation Fibrinolysis. 1991;2:69–75. [PubMed] [Google Scholar]
- 11.Markwardt F. Pharmazie. 1994;49:313–316. [PubMed] [Google Scholar]
- 12.Rydel T J, Ravichandran K G, Tulinsky A, Bode W, Huber R, Roitsch C, Fenton J W, II. Science. 1990;249:277–280. doi: 10.1126/science.2374926. [DOI] [PubMed] [Google Scholar]
- 13.Grütter M G, Priestle J P, Rahuel J, Grossenbacher H, Bode W, Hofsteenge J, Stone S R. EMBO J. 1990;9:2361–2365. doi: 10.1002/j.1460-2075.1990.tb07410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Locht A, Lamba D, Bauer M, Huber R, Friedrich T, Kröger B, Höffken W, Bode W. EMBO J. 1995;14:5149–5157. doi: 10.1002/j.1460-2075.1995.tb00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Locht A, Stubbs M T, Bode W, Friedrich T, Bollschweiler C, Höffken W, Huber R. EMBO J. 1996;15:6011–6017. [PMC free article] [PubMed] [Google Scholar]
- 16.Noeske-Jungblut Ch, Haendler B, Donner P, Alagon A, Possani L, Schleuning W-D. J Biol Chem. 1995;270:28629–28634. doi: 10.1074/jbc.270.48.28629. [DOI] [PubMed] [Google Scholar]
- 17.Glusa E., Bretschneider, E., Danm, J. & Noeske-Jungblut, C. (1997) Thromb. Haemostasis, in press. [PubMed]
- 18.Petri T, Neukamm B, Isernhagen M, Ockert B, Noeske-Jungblut C. In: Animal Cell Technology: From Vaccines to Genetic Medicine. Carrondo M J, Griffiths B, Moreira J L P, editors. Dordrecht, The Netherlands: Kluwer; 1997. pp. 525–528. [Google Scholar]
- 19.Brandstetter H, Turk D, Höffken H W, Grosse D, Stürzebecher J, Martin P D, Edwards B F P, Bode W. J Mol Biol. 1992;226:1085–1099. doi: 10.1016/0022-2836(92)91054-s. [DOI] [PubMed] [Google Scholar]
- 20.Leslie A G W. mosflm User Guide. Cambridge, U.K.: Medical Research Council Laboratory of Molecular Biology; 1994. , Version 5.20. [Google Scholar]
- 21.Collaborative Computational Project, No. 4. Acta Crystallogr D. 1994;50:760–763. [Google Scholar]
- 22.Navaza J. Acta Crystallogr A. 1994;50:157–163. [Google Scholar]
- 23.Turk D. Ph.D. thesis. Munich: Technische Universität; 1992. [Google Scholar]
- 24.Brünger A T, Kuriyan K, Karplus M. Science. 1987;235:458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- 25.Engh R A, Huber R. Acta Crystallogr A. 1991;47:392–400. [Google Scholar]
- 26.Laskowski R A, MacArthur M W, Moss D S, Thornton J M. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 27.Lessel U, Schomburg D. Protein Eng. 1994;7:1175–1187. doi: 10.1093/protein/7.10.1175. [DOI] [PubMed] [Google Scholar]
- 28.Jones T A. J Appl Crystallogr. 1987;11:268–272. [Google Scholar]
- 29.Huber R, Schneider M, Mayr I, Müller R, Deutzmann R, Suter F, Zuber H, Falk H, Kayser H. J Mol Biol. 1987;198:499–513. doi: 10.1016/0022-2836(87)90296-8. [DOI] [PubMed] [Google Scholar]
- 30.Zanotti G, Monaco H L. J Mol Biol. 1993;230:613–624. doi: 10.1006/jmbi.1993.1173. [DOI] [PubMed] [Google Scholar]
- 31.Padmanabhan K, Padmanabhan K P, Tulinsky A, Park C, Bode W, Huber R, Blankenship D T, Cardin A D, Kisiel W. J Mol Biol. 1993;232:947–966. doi: 10.1006/jmbi.1993.1441. [DOI] [PubMed] [Google Scholar]
- 32.Bode W, Huber R. Eur J Biochem. 1992;204:433–451. doi: 10.1111/j.1432-1033.1992.tb16654.x. [DOI] [PubMed] [Google Scholar]
- 33.Flower D R, North A C T, Atwood T K. Protein Sci. 1993;2:753–761. doi: 10.1002/pro.5560020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flower D R. Biochem J. 1996;318:1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newcomer M E, Jones T A, Åqvist J, Sundelin J, Eriksson U, Rask I, Peterson P A. EMBO J. 1984;3:1451–1454. doi: 10.1002/j.1460-2075.1984.tb01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernstein F C, Koetzle T F, Wiliams G J B, Meyer E F, Jr, Brice M D, Rodgers J R, Kennard O, Shimanouchi T, Tasumi M. J Mol Biol. 1977;112:535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- 37.Tegoni M, Ramoni R, Bignetti E, Spinelli S, Cambillau C. Nat Struct Biol. 1996;3:863–867. doi: 10.1038/nsb1096-863. [DOI] [PubMed] [Google Scholar]
- 38.Böcskei Z, Groom C R, Flower D R, Wright C E, Phillips S E V, Cavaggioni A, Findlay J B C, North A C T. Nature (London) 1992;360:186–188. doi: 10.1038/360186a0. [DOI] [PubMed] [Google Scholar]
- 39.Holden H M, Rypniewski W R, Law J H, Rayment I. EMBO J. 1987;6:1565–1570. doi: 10.1002/j.1460-2075.1987.tb02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newcomer M E. Structure. 1993;1:7–18. doi: 10.1016/0969-2126(93)90004-z. [DOI] [PubMed] [Google Scholar]
- 41.Barton G J. Protein Eng. 1993;6:37–40. doi: 10.1093/protein/6.1.37. [DOI] [PubMed] [Google Scholar]
- 42.Noeske-Jungblut C, Krätzschmar J, Haendler B, Alagon A, Possani L, Verhallen P, Donner P, Schleuning W-D. J Biol Chem. 1994;269:5050–5053. [PubMed] [Google Scholar]
- 43.Wu Q, Sheehan J P, Tsiang M, Lentz S R, Birktoft J J, Sadler J E. Proc Natl Acad Sci USA. 1991;88:6775–6779. doi: 10.1073/pnas.88.15.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsiang M, Jain A K, Dunn K E, Rojas M E, Leung L L K, Gibbs C S. J Biol Chem. 1995;270:16854–16863. doi: 10.1074/jbc.270.28.16854. [DOI] [PubMed] [Google Scholar]
- 45.Mathews I I, Padmanabhan K P, Tulinsky A, Sadler J E. Biochemistry. 1994;33:13547–13552. doi: 10.1021/bi00250a006. [DOI] [PubMed] [Google Scholar]
- 46.Mattews I I, Padmanabhan K P, Ganesh V, Tulinsky A, Ishii M, Chen J, Turck C W, Coughlin S R, Fenton J W, II. Biochemistry. 1994;33:3266–3279. doi: 10.1021/bi00177a018. [DOI] [PubMed] [Google Scholar]
- 47.Banfield D K, MacGillivray R T A. Proc Natl Acad Sci USA. 1992;89:2779–2783. doi: 10.1073/pnas.89.7.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]