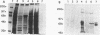Abstract
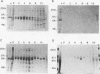
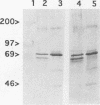
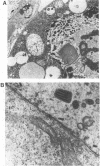
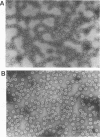
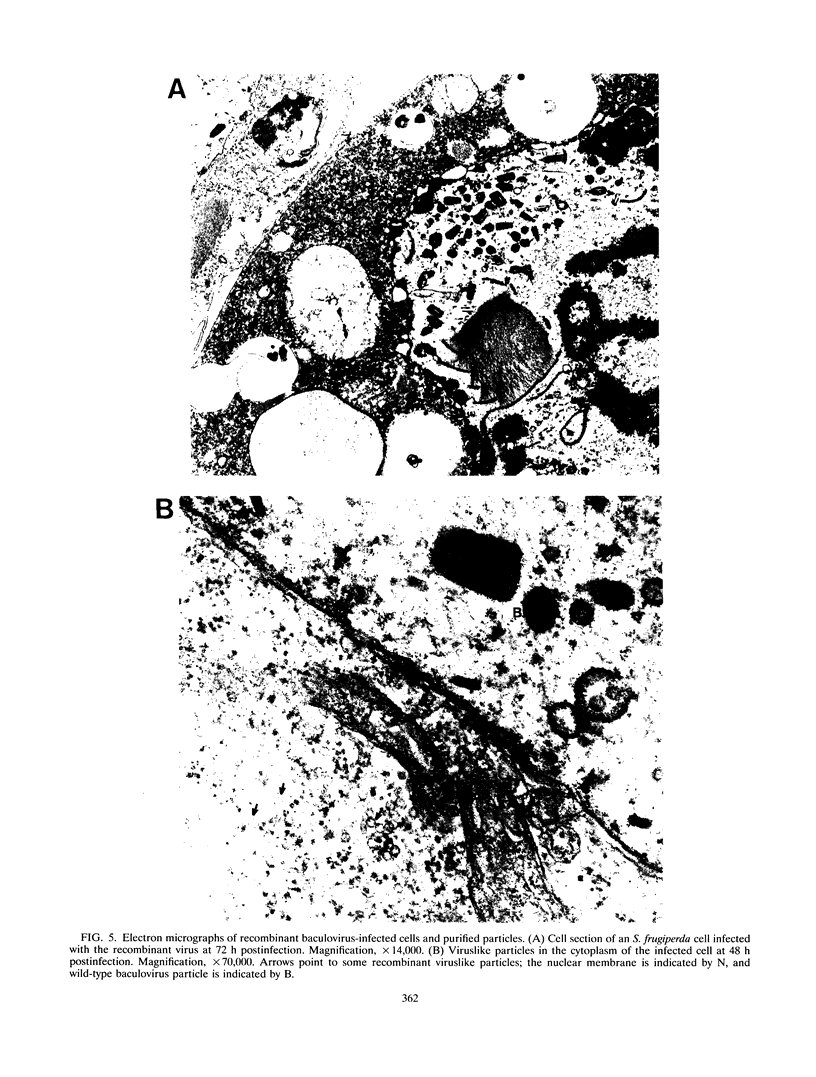
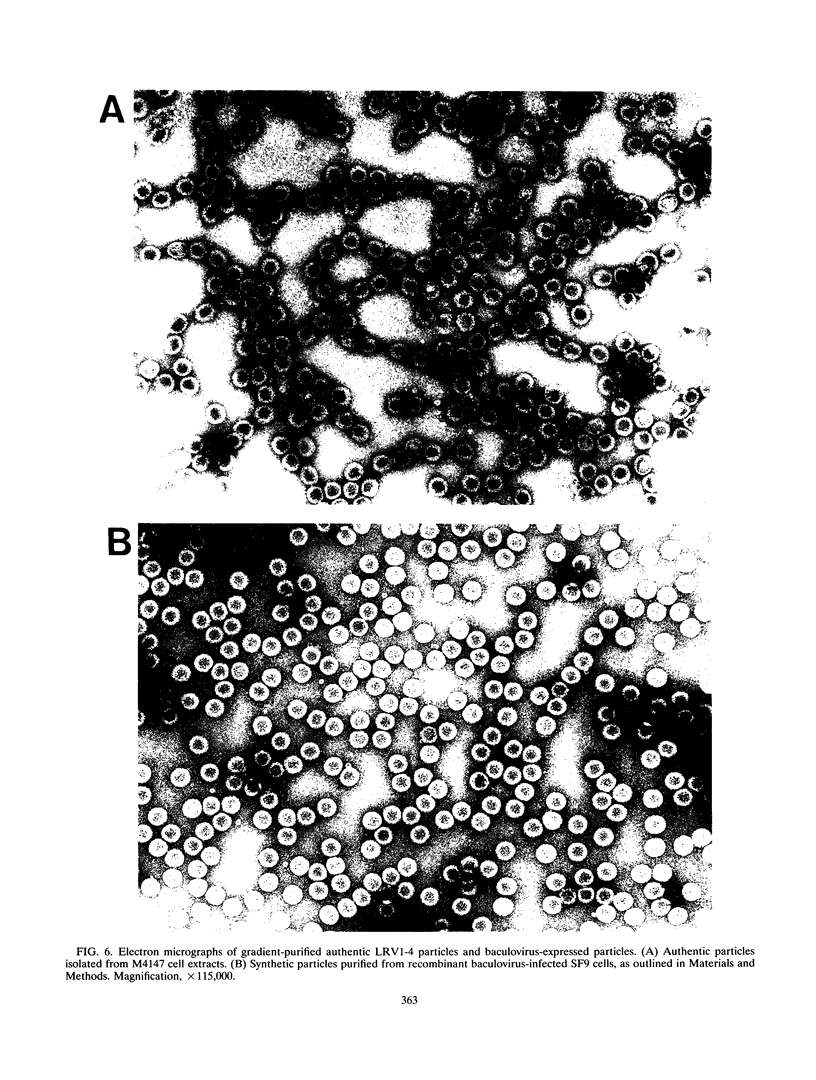
The putative capsid open reading frame (ORF2) of the Leishmania RNA virus LRV1-4 was expressed in a baculovirus expression system. The expressed protein was identified by Western immunoblot analysis with polyclonal antiserum raised to purified LRV1-4 virus. Electron microscopy and sedimentation analysis indicated that the expressed protein self-assembles into empty viruslike particles of similar size and shape to authentic virus particles, thus confirming that ORF2 encodes the viral capsid. The expressed particles are present exclusively in the cytoplasm of infected SF9 cells and are able to assemble in the absence of LRV1-4 RNA, viral polymerase, or any Leishmania host factors.
Full text
PDF

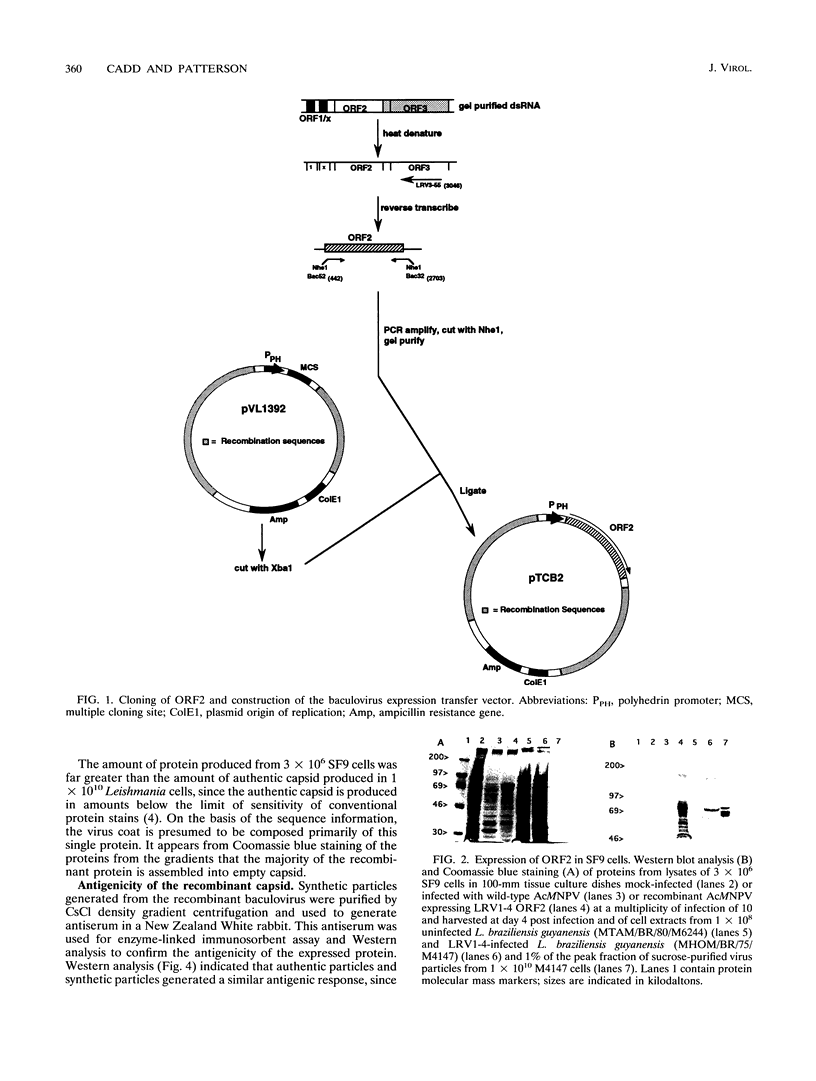


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong T. C., Keenan M. C., Widmer G., Patterson J. L. Successful transient introduction of Leishmania RNA virus into a virally infected and an uninfected strain of Leishmania. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1736–1740. doi: 10.1073/pnas.90.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellofatto V., Cross G. A. Expression of a bacterial gene in a trypanosomatid protozoan. Science. 1989 Jun 9;244(4909):1167–1169. doi: 10.1126/science.2499047. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A. Relationships among the positive strand and double-strand RNA viruses as viewed through their RNA-dependent RNA polymerases. Nucleic Acids Res. 1991 Jan 25;19(2):217–226. doi: 10.1093/nar/19.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadd T. L., Keenan M. C., Patterson J. L. Detection of Leishmania RNA virus 1 proteins. J Virol. 1993 Sep;67(9):5647–5650. doi: 10.1128/jvi.67.9.5647-5650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. S., Mattern C. F. Protozoal viruses. Adv Virus Res. 1976;20:87–112. doi: 10.1016/s0065-3527(08)60502-3. [DOI] [PubMed] [Google Scholar]
- Dinman J. D., Icho T., Wickner R. B. A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French T. J., Roy P. Synthesis of bluetongue virus (BTV) corelike particles by a recombinant baculovirus expressing the two major structural core proteins of BTV. J Virol. 1990 Apr;64(4):1530–1536. doi: 10.1128/jvi.64.4.1530-1536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbride L., Myler P. J., Stuart K. Distribution and sequence divergence of LRV1 viruses among different Leishmania species. Mol Biochem Parasitol. 1992 Aug;54(1):101–104. doi: 10.1016/0166-6851(92)90099-6. [DOI] [PubMed] [Google Scholar]
- Icho T., Wickner R. B. The double-stranded RNA genome of yeast virus L-A encodes its own putative RNA polymerase by fusing two open reading frames. J Biol Chem. 1989 Apr 25;264(12):6716–6723. [PubMed] [Google Scholar]
- Jagadish M. N., Ward C. W., Gough K. H., Tulloch P. A., Whittaker L. A., Shukla D. D. Expression of potyvirus coat protein in Escherichia coli and yeast and its assembly into virus-like particles. J Gen Virol. 1991 Jul;72(Pt 7):1543–1550. doi: 10.1099/0022-1317-72-7-1543. [DOI] [PubMed] [Google Scholar]
- Jiang X., Wang M., Graham D. Y., Estes M. K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992 Nov;66(11):6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler G. M., Beverley S. M. Transcriptional mapping of the amplified region encoding the dihydrofolate reductase-thymidylate synthase of Leishmania major reveals a high density of transcripts, including overlapping and antisense RNAs. Mol Cell Biol. 1989 Sep;9(9):3959–3972. doi: 10.1128/mcb.9.9.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laban A., Wirth D. F. Transfection of Leishmania enriettii and expression of chloramphenicol acetyltransferase gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9119–9123. doi: 10.1073/pnas.86.23.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon P. T., Hirasawa T., Oldfield S., Murphy M., Roy P. Expression of the outer capsid protein VP5 of two bluetongue viruses, and synthesis of chimeric double-shelled virus-like particles using combinations of recombinant baculoviruses. Virology. 1991 Jun;182(2):793–801. doi: 10.1016/0042-6822(91)90620-q. [DOI] [PubMed] [Google Scholar]
- Loudon P. T., Roy P. Assembly of five bluetongue virus proteins expressed by recombinant baculoviruses: inclusion of the largest protein VP1 in the core and virus-like proteins. Virology. 1991 Feb;180(2):798–802. doi: 10.1016/0042-6822(91)90094-r. [DOI] [PubMed] [Google Scholar]
- Patterson J. L. Viruses of protozoan parasites. Exp Parasitol. 1990 Jan;70(1):111–113. doi: 10.1016/0014-4894(90)90091-p. [DOI] [PubMed] [Google Scholar]
- Ruffing M., Zentgraf H., Kleinschmidt J. A. Assembly of viruslike particles by recombinant structural proteins of adeno-associated virus type 2 in insect cells. J Virol. 1992 Dec;66(12):6922–6930. doi: 10.1128/jvi.66.12.6922-6930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart K. D., Weeks R., Guilbride L., Myler P. J. Molecular organization of Leishmania RNA virus 1. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8596–8600. doi: 10.1073/pnas.89.18.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr P. I., Aline R. F., Jr, Smiley B. L., Scholler J., Keithly J., Stuart K. LR1: a candidate RNA virus of Leishmania. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9572–9575. doi: 10.1073/pnas.85.24.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercero J. C., Wickner R. B. MAK3 encodes an N-acetyltransferase whose modification of the L-A gag NH2 terminus is necessary for virus particle assembly. J Biol Chem. 1992 Oct 5;267(28):20277–20281. [PubMed] [Google Scholar]
- Valentine R. C., Green N. M. Electron microscopy of an antibody-hapten complex. J Mol Biol. 1967 Aug 14;27(3):615–617. doi: 10.1016/0022-2836(67)90063-0. [DOI] [PubMed] [Google Scholar]
- Weeks R. S., Patterson J. L., Stuart K., Widmer G. Transcribing and replicating particles in a double-stranded RNA virus from Leishmania. Mol Biochem Parasitol. 1992 Jun;52(2):207–213. doi: 10.1016/0166-6851(92)90053-m. [DOI] [PubMed] [Google Scholar]
- Widmer G., Comeau A. M., Furlong D. B., Wirth D. F., Patterson J. L. Characterization of a RNA virus from the parasite Leishmania. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5979–5982. doi: 10.1073/pnas.86.15.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]