Abstract
Comparative morphological and functional analyses of the skeletal remains of Oreopithecus bambolii, a hominoid from the Miocene Mediterranean island of Tuscany–Sardinia (Italy), provides evidence that bipedal activities made up a significant part of the positional behavior of this primate. The mosaic pattern of its postcranial morphology is to some degree convergent with that of Australopithecus and functionally intermediate between apes and early hominids. Some unique traits could have been selected only under insular conditions where the absence of predators and the limitation of trophic resources play a crucial role in mammalian evolution.
Keywords: Miocene, bipedality, insularity
Oreopithecus bambolii (1) is an Upper Miocene (Turolian, 9–7 my) (2) large-bodied hominoid. Known remains come from lacustrine sediments of Tuscany (the old lignite mines of Monte Bamboli, Casteani, Ribolla, and Baccinello; ref. 3) and more recently from Sardinian fluviatile deposits of similar age (4).
Oreopithecus shows a series of morphological features typical of living apes that reflect significant adaptations to vertical climbing, in particular the wide thorax, the short trunk, and the high intermembral index (3, 5–7).
Nevertheless, in the late 1950s and 1960s some Oreopithecus characters (high epicondylar angle of the distal femur, relatively short hip bone, reduced distance between insertion area of the sacrum on the ilium and acetabulum, and a hominid-like antero-inferior iliac spine) were linked to bipedality (3, 5, 8, 9). However, the few characters, and the insufficiently restored material, have not been convincing enough for such an unorthodox idea to be accepted. As a consequence, the bipedality hypothesis has not been discussed seriously since; the characters at issue were interpreted as adaptations to vertical climbing and asserted to be present in the last common ancestor of living hominoids and primitively retained in Oreopithecus (10).
Over the last 2 years we restored a large portion of the unpublished material from collections of the Naturhistorisches Museum in Basel, Switzerland and found several unidentified specimens. The detailed study of this material demonstrated a series of important characters. From this base, we want to reopen the issue and discuss whether these and other previously undescribed characters are present in extant apes, whether they are shared with and retained from the contemporaneous Dryopithecus, suggested to be sister to Oreopithecus (11) and considered as a vertical climber and suspensory ape (12), or if some or all of these features are instead related to bipedal activities.
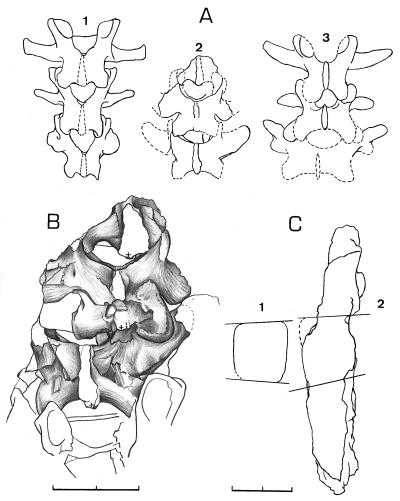
The lumbar region of Oreopithecus comprises five vertebrae (6, 7, 13). The restored specimen BA72 shows several characters indicating a lumbar lordosis. Both the human-like vertebral wedging pattern (ref. 14; Table 1; Fig. 1C2) and the caudally progressive increase of the interfacet distances (Table 2; Fig. 1 A2 and B) are direct evidence of lordosis and considered a uniquely hominid condition (15). The associated caudal surface area increase of the laminae (similar to the pattern of Australopithecus and Homo) also is functionally concordant with the structural and mechanical demands of a lordotic lumbar region. Dryopithecus is not lordotic, as it has the wedging pattern of Pan and Hylobates (L5 index 87; Table 1; Fig. 1C1) and a caudally progressive decrease of the interfacet distances.
Figure 1.
The lumbar region of Oreopithecus. Dorsal view of the lumbar region. (A1) Ape pattern with a caudally progressive decrease of the interfacet distances (Pan troglodytes, wild shot). (A2) Oreopithecus reconstructed, showing increasing distances from L3 to L5. (A3) Hominid pattern with a caudally progressive increase of the interfacet distances [Australopithecus, after Robinson (33)]. (B) Dorsal view of the Oreopithecus lumbar region with L3-L5 (BA72, + marks the center of the arch). (C1) Sagittal section of L5 of Dryopithecus laietanus. (CLL-18800, left ventral, right dorsal). (C2) Sagittal section of the lumbar region of Oreopithecus (BA72, left ventral, right dorsal). (BA = Naturhistorisches Museum in Basel, Switzerland, which provided A and B.) (Scale = 2 cm.)
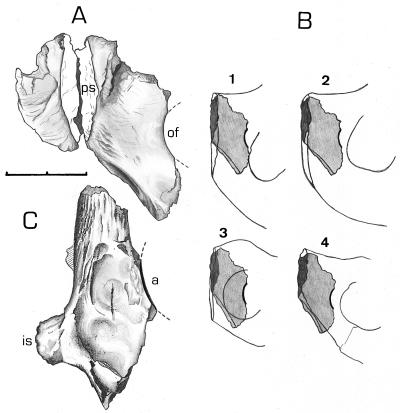
The anatomy of pubis and ischium of Oreopithecus is not ape-like, but the pubis closely resembles in size and shape that of Australopithecus afarensis (Fig. 2 A and B). Restored, the specimen BA71 exposes the medial border of the obturator foramen, demonstrating a previously unrecognized extreme shortness of the pubic symphysis (16) and a straight inferior margin of the pubis. This specimen retains a surprisingly short ischium. A further previously undescribed partial ischium with acetabulum (BA182, Fig. 2C) exhibits an extraordinarily large ischial spine (present and of similar size and form in two other partial ischia, BA71 and BA208, although less well preserved). This spine is much larger than in apes, but identical to that of Homo (17).
Figure 2.
Pelvic elements of O. bambolii. (A) Complete pubic symphysis (BA71), dorsal view. ps, pubic symphysis; of, obturator foramen. (B) Dorsal view of the left partial pubis of Oreopithecus, drawn to the same scale and overlapping those of (1) Pan, (2) Pongo, (3) Symphalangus, and (4) A. afarensis (AL 288–1). (C) Partial ischium (BA71) with acetabulum (a) and ischial spine (is). The ischial spine is only weakly developed in living apes and is associated with the diaphragma that joins the sacrospinous ligament, stressing it when supporting the viscera during vertical postures of the trunk (17). In bipedal hominids, however, the ischial spine is very large, due to the increased loading and strength of the sacrospinous ligament, which prevents rotation of the sacrum under the load of the trunk (34). (Scale = 2 cm.)
The anatomy of the distal femora of Oreopithecus, previously interpreted as evidence of a carrying angle (9), alternatively has been related functionally to vertical climbing (5, 10). Humans have a genu valgum (diaphyseal angle on the distal femora that places the knee joints close to the line of gravity to support and transmit body weight during bipedal locomotion) as a consequence of increased bone deposition on the medial side of the diaphysis. The occasionally observable moderate “obliquity” of the femora of Pongo and Hylobates is due rather to the greater height of the medial condyle and is restricted exclusively to the epiphysis (18). These two contrasting patterns reflect the different functional demands of bipedality and vertical climbing (18). The femur of Oreopithecus shows a pronounced diaphyseal angle combined with condyles of subequal size, similar to Australopithecus and Homo and functionally correlated with bipedal activities.
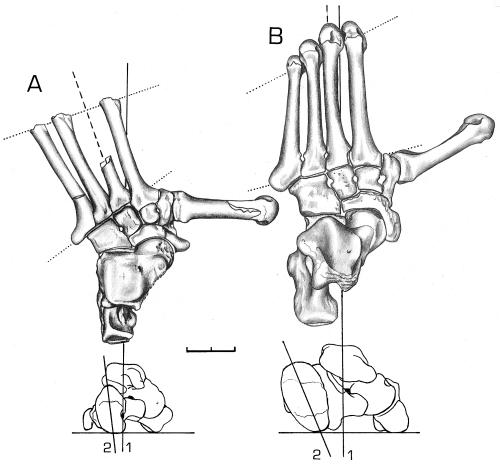
The Oreopithecus foot, previously described as chimp-like (19), differs from the latter considerably (Fig. 3) as the design is unique among primates in its structure and function. Most striking is the deviation of the line of leverage from the habitual primate direction parallel to the third metatarsal; in Oreopithecus this falls between the first and second metatarsals. This is due to a permanent abduction of the lateral metatarsals that are thus fixed in that posture gorillas habitually use when on the ground. The small size of the cuboid, the large joint surfaces of the lateral and central cuneiforms, and the Mt2 firmly embedded between all cuneiforms and the Mt3, indicate increased force transmission on the medial side of the foot. This is in keeping with the medially deviated line of leverage and in contrast to the general emphasis on the lateral side as in all other primates. The metatarsals are short and only minimally curved, and their lengths decrease medially until subequal. This, together with the change in direction of leverage, reduces bending moments especially in those metatarsals that are commonly aligned with the direction of travel during terrestrial locomotion. At the same time this increases the area of support of the foot for balance during displacements of the center of gravity in bipedal standing and walking. The orientation of the tuber calcis nearly vertical to the ground and at a right angle to the proximal talar surface (Fig. 3, Lower), as well as the similar height of the medial and lateral border of the talus, suggest that the axis of the tibia was in line with the line of gravity. This morphology is concordant with the inferred genu valgum and is biomechanically linked to sagittal movements. In contrast, the lateral border of the Dryopithecus talus is much higher than the medial one and thus indicative of a genu varum (femora diverge distally so that the knee joints are far from the line of gravity, allowing optimal positioning of the legs during vertical climbing). Some further characters appreciably reduce the range of mobility and grasping capability of the foot. The calcaneo-cuboid joint is very low and rectangular, contrary to the general primate pattern, indicating little, if any, dorsiflexion and no rotatory movement. The metatarso-cuboid joint surfaces are even flatter than in Papio and thus minimize movement. The MtV-cuboid contact differs from that of other primates, including Dryopithecus, as the cuboid lacks the lateral tilt of the joint surface that articulates with the tuberosity of the fifth metatarsal. Thus, this joint cannot transmit support reaction forces acting on the lateral border of the foot when this is inverted during vertical climbing.
Figure 3.
Foot morphology. (A) Oreopithecus (based on BA79 and BA83, right and left foot of the same individual). (B) Pan. (Upper) Dorsal view. Continuous line, long axis of the foot; interrupted line, axis of Mt3; lower dotted line, tarso-metatarsal joint axis, showing the permanent abduction of the lateral metatarsals; upper dotted line joining the distal ends of the Mt2–5 diaphyses, showing the medially decreasing length of the metatarsals. (Lower) Posterior view of the articulated tarsal elements. 1, Line of gravity; 2, inclination of the tuber calcis. (Scale = 2 cm.)
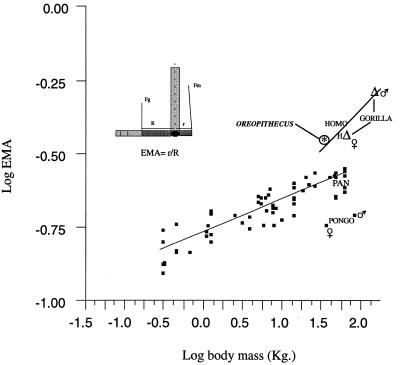
Foot proportions (power arm/load arm ratio) of primates reflect body weight carried by the feet (Fig. 4). Platyrrhines, cercopithecids, and chimpanzees show an allometric relationship with a slope 0.11 (r = 0.933). This means that the proportions of the primates’ feet are correlated with body weight. Relative to this baseline, two divergent patterns emerge. The slow climber and suspensor (Pongo) shows elongation of the metatarsals and phalanges, adaptations for more effective hanging. The feet hence lose most of their propulsive functions on the ground or on large-diameter supports. Gorilla and Homo show an opposite pattern, increasing their power arm/load arm ratio, although for different reasons. In Gorilla, this is due to the enormous body mass. The ability of muscles to generate force does not increase isometrically with their mass but with their cross-section (20). Therefore, the force of the muscle increases less than does body mass (21). Extremely big animals such as Gorilla reach a limit where they have to compensate for this relative loss of muscle force by improving the lever advantage for these muscles. This, however, is possible only in those animals that do not need speed (22). In humans, the increase in power arm/load arm ratio is due to bipedality, because the feet have to support the entire body weight. This requires a change of proportions to maintain peak bone stresses similar to those of quadrupeds (21). The foot proportions of Oreopithecus contrast with those of specialized climbers, nor do they match those of platyrrhines or cercopithecids, but rather fall close to the unusual proportions shown by Gorilla and Homo. Due to its low body mass, Oreopithecus is not comparable with Gorilla; however, striking similarities with humans exist regarding the relation of foot proportions and body mass.
Figure 4.
The foot proportions of Oreopithecus. A calculation of the power arm/load arm ratio (EMA) (21) for a broad spectrum of anthropoids (platyrrhines, cercopithecids, and hominoids). We measured R (load arm) from the head of MT 3 to the center of the trochlear surface of the talus, and r (power arm) from the latter to the distal part of the tuber calcis. Values for Gorilla, Homo, and Pongo are an average of body weight classified by sex; for Oreopithecus, the body weight is that estimated for the male individual IGF 11778 (32 kg) (35), whose size is consistent with that of the foot. Platyrrhines, cercopithecids, and chimpanzees show an allometric relationship with a slope 0.11. r = 0.933. IGF = Institute of Geology of Florence.
Compared with the postcranial anatomy of the suspensory vertical climber and hypothetical ancestor, Dryopithecus, we interpret these many morphological features of Oreopithecus as autapomorphies and not as primitive retentions. None of the extant or fossil apes shows these characters. They are found elsewhere exclusively in hominids and are considered to be adaptations to static and mechanical demands of bipedal postures and locomotion. This suggests that bipedal activities made up a significant part of the positional behavior of Oreopithecus, at least as much as to have been reflected in these many features of skeletal design.
As in Australopithecus, Oreopithecus shows a mosaic of primitive (ape-like) and derived (hominid-like) features, but the resulting morphological pattern is different, because the paleoenvironmental scenario and the ancestral condition are not the same. Oreopithecus is an endemic primate from an Upper Miocene Mediterranean island. Fossil insular ecosystems are well studied, especially Mediterranean ones (23). They are characterized by lack of predators and limitation of space and thus of trophic resources (23, 24). Whereas the absence of predation removes the need for adaptations related to predator avoidance, intraspecific and interspecific competition for food resources increases (23, 24). Both factors impose specific selective pressures that favor, on the one hand, adaptations linked to low energy expenditure, namely those related to energetically less expensive locomotor activities (flightless birds, ref. 25), and to reduction of bone mass in the locomotor apparatus at the expense of mobility and speed (26) (reduction of limb lengths in all mammals, fusion of limb elements in ruminants, elephants, and hippos, ref. 23). On the other hand, they select for feeding strategies that increase the efficiency of resource utilization (increase in hypsodonty, rodent-like continuously growing incisors in bovids, reduction of premolars in many groups, etc.) (23). These adaptations are universally found in all mammal faunas of small islands.
These selective pressures probably played a crucial role in the evolution of Oreopithecus, too, because the accompanying bovid fauna clearly exhibits the typical traits of insularity (27), such as strongly reduced limb bones and continuously growing incisors (28). In Oreopithecus, the lack of predators may have led to a decrease of energetically expensive (29, 30) and risky (31) climbing activities, while favoring significant terrestriality. Bipedal standing while foraging, combined with bipedal shuffling during frequent short distance travel during food gathering, as recently described for wild chimpanzees (32), could have increased the harvesting efficiency for this ape. The postcranial morphology of Oreopithecus clearly reflects such bipedal terrestrial activities. The peculiar feet, less suitable for fast walking or running than those of early hominids, yield, however, an especially well designed platform for stable postural harvesting, as the tripod formed by the deviated metatarsals and the widely abducted hallux provides a large area of support. Short legs further increase stability during bipedal stance because the center of gravity is low. Both features, short legs and short lever arm of the feet, indicate short stride length and low speed and suggest bipedal shuffling.
The morphology of Oreopithecus is not ape-like, because it is functionally designed for habitual and not facultative terrestrial bipedal activities, but neither is it hominid-like, as the special environmental conditions of islands engraved their peculiar traits. Nevertheless, the striking convergences with and differences from hominids clearly make Oreopithecus a key species for understanding human bipedality.
Table 1.
Vertebral wedging calculated by the ratio ventral to dorsal length of the vertebral bodies
| Sex | n | Mean | Range | SD | |
|---|---|---|---|---|---|
| Homo sapiens | F | 10 | 106 | 98–114 | 5 |
| M | 10 | 104 | 96–113 | 4 | |
| Oreopithecus bambolii | F? | 1 | 108 | ||
| Dryopithecus laietanus | M | 1 | 87 | ||
| Pan troglodytes | F | 9 | 93 | 83–99 | 6 |
| M | 9 | 93 | 80–99 | 7 | |
| Gorilla gorilla | F | 10 | 99 | 96–103 | 3 |
| M | 10 | 95 | 88–99 | 4 | |
| Hylobates sp. | F | 5 | 94 | 93–97 | 2 |
| M | 8 | 92 | 85–98 | 7 |
Data from ref. 14.
Table 2.
Interfacet distances (Id) in adult lumbar vertebrae
| Spinal level |
Homo sapiens,*n = 30
|
Homo erectus KNM-WT 15000, n = 1
|
Oreopithecus bambolii BA72, n = 1
|
Pan troglodytes, n = 16
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Id | Mean | Id | Mean | Id | Mean | SD | Id | |
| 2 | 32.51 | 3.88 | +9 | 31.5 | +23 | 23.0 | 27.02 | 2.54 | −0 | |
| 1 | 37.43 | 3.49 | +13 | 32.0 | +1 | 23.6 | +3 | 25.85 | 2.59 | −1 |
| 0 | 43.76 | 2.72 | +14 | 33.5 | +4 | 25.4 | +7 | 23.74 | 2.72 | −2 |
Except Oreopithecus the data come from ref. 15. Index of difference in interfacet distances (mm): Id = Inferior d. − superior d./Inferior d. × 100. In the case of Oreopithecus the interfacet distance is measured at the external border of the postzygapophyses.
Specimens with five lumbar vertebrae (0 = first sacral vertebrae; 1 = first presacral vertebrae, numbers increase in a cranial direction).
Acknowledgments
We gratefully thank B. Engesser for permission to study the Oreopithecus specimens from Basel and for the loan of material. We thank M. Pickford, H. Preuschoft, L. Rook, B. Wood, D. Pilbeam, T. White, and three anonymous reviewers for helpful comments and P. Schmid, J. Barreiros, and F. Uribe for allowing the study of material in their care. We are grateful to D. Oppliger for his help in Basel and to I. Pellejero for professional cleaning and casting of the fossil material. We are especially grateful to J. Hürzeler, who risked his life collecting the material. This work was partially supported by the Wenner Gren Foundation.
References
- 1.Gervais P. C R Acad Sci Paris. 1872;74:1217–1223. [Google Scholar]
- 2.Engesser B. Bull Soc Paleontol Ital. 1989;28:226–252. [Google Scholar]
- 3.Hürzeler J. Schweiz palaeont Abh. 1949;66:1–20. [Google Scholar]
- 4.Cordy J M, Ginesu S. C R Acad Sci Paris. 1994;318:697–704. [Google Scholar]
- 5.Straus W L. In: Classification and Human Evolution. Washburn S L, editor; Washburn S L, editor. Chicago: Aldine; 1963. pp. 146–177. [Google Scholar]
- 6.Harrison T. J Hum Evol. 1987;15:541–583. [Google Scholar]
- 7.Sarmiento E. Am Mus Novit. 1987;2881:1–44. [Google Scholar]
- 8.Straus W L. Clin Orthop. 1962;25:9–19. [PubMed] [Google Scholar]
- 9.Kummer B. Mitt Naturforsch Ges. 1965;22:239–259. [Google Scholar]
- 10.Harrison T. In: Origine(s) de la Bipedie Chez les Hominidés. Senut B, Coppens Y, editors; Senut B, Coppens Y, editors. Paris: Centre National de la Recherche Scientifique; 1991. pp. 235–244. [Google Scholar]
- 11.Moyà-Solà S, Köhler M. C R Acad Sci Paris. 1997;324:141–148. [Google Scholar]
- 12.Moyà-Solà S, Köhler M. Nature (London) 1996;379:156–159. doi: 10.1038/379156a0. [DOI] [PubMed] [Google Scholar]
- 13.Schultz A H. Z Morphol Anthropol. 1960;50:136–149. [Google Scholar]
- 14.Sanders W J, Bodenbender B E. J Hum Evol. 1994;26:203–238. [Google Scholar]
- 15.Latimer B, Ward C. In: The Nariokotome Homo erectus Skeleton. Walker A, Leakey A, editors; Walker A, Leakey A, editors. Berlin: Springer; 1993. pp. 266–293. [Google Scholar]
- 16.Hürzeler J. Ann Paleont. 1968;54:195–233. [Google Scholar]
- 17.Abitbol M M. Am J Phys Anthropol. 1988;75:53–67. doi: 10.1002/ajpa.1330750107. [DOI] [PubMed] [Google Scholar]
- 18.Preuschoft H, Tardieu C. Folia Primatol. 1996;66:82–92. doi: 10.1159/000157187. [DOI] [PubMed] [Google Scholar]
- 19.Szalay F S, Langdon J. J Hum Evol. 1987;15:585–621. [Google Scholar]
- 20.Starck D. Vergleichende Anatomie der Wirbeltiere. Vol. 2. Berlin: Springer; 1979. pp. 3–776. [Google Scholar]
- 21.Biewener A A. Science. 1989;245:45–48. doi: 10.1126/science.2740914. [DOI] [PubMed] [Google Scholar]
- 22.Preuschoft H. Z Anat Entwicklungsgesch. 1970;131:156–192. [PubMed] [Google Scholar]
- 23.Sondaar P. In: Major Patterns of Vertebrate Evolution. Hecht M K, Goody P C, Hecht B M, editors; Hecht M K, Goody P C, Hecht B M, editors. New York: Plenum; 1977. pp. 671–707. [Google Scholar]
- 24.MacArthur R H, Wilson E O. The Theory of Island Biogeography. Princeton: Princeton Univ. Press; 1967. pp. 1–130. [Google Scholar]
- 25.James F H, Olson S L. Nat Hist. 1983;92:30–40. [Google Scholar]
- 26.Currey J. The Mechanical Adaptation of Bones. Princeton: Princeton Univ. Press; 1984. pp. 1–294. [Google Scholar]
- 27.Hürzeler J, Engesser B. C R Acad Sci Paris. 1976;283:333–336. [Google Scholar]
- 28.Hürzeler J. C R Acad Sci Paris. 1983;296:497–503. [Google Scholar]
- 29.Persons P E, Taylor C R. Physiol Zool. 1977;50:107–117. [Google Scholar]
- 30.Yamazaki N, Ishida H. J Hum Evol. 1983;8:337–349. [Google Scholar]
- 31.Schultz A H. Contrib Embryol. 1941;182:58–111. [Google Scholar]
- 32.Hunt K D. J Hum Evol. 1994;26:183–202. [Google Scholar]
- 33.Robinson J T. Early Hominid Posture and Locomotion. Chicago: Univ. of Chicago Press; 1972. pp. 1–361. [Google Scholar]
- 34.Kapandji I A. Cuadernos de Fisiologia Articular. Paris: Masson; 1994. pp. 1–255. [Google Scholar]
- 35.Jungers W L. J Hum Evol. 1987;16:445–456. [Google Scholar]