Abstract
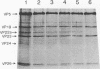
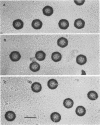
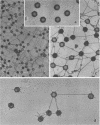
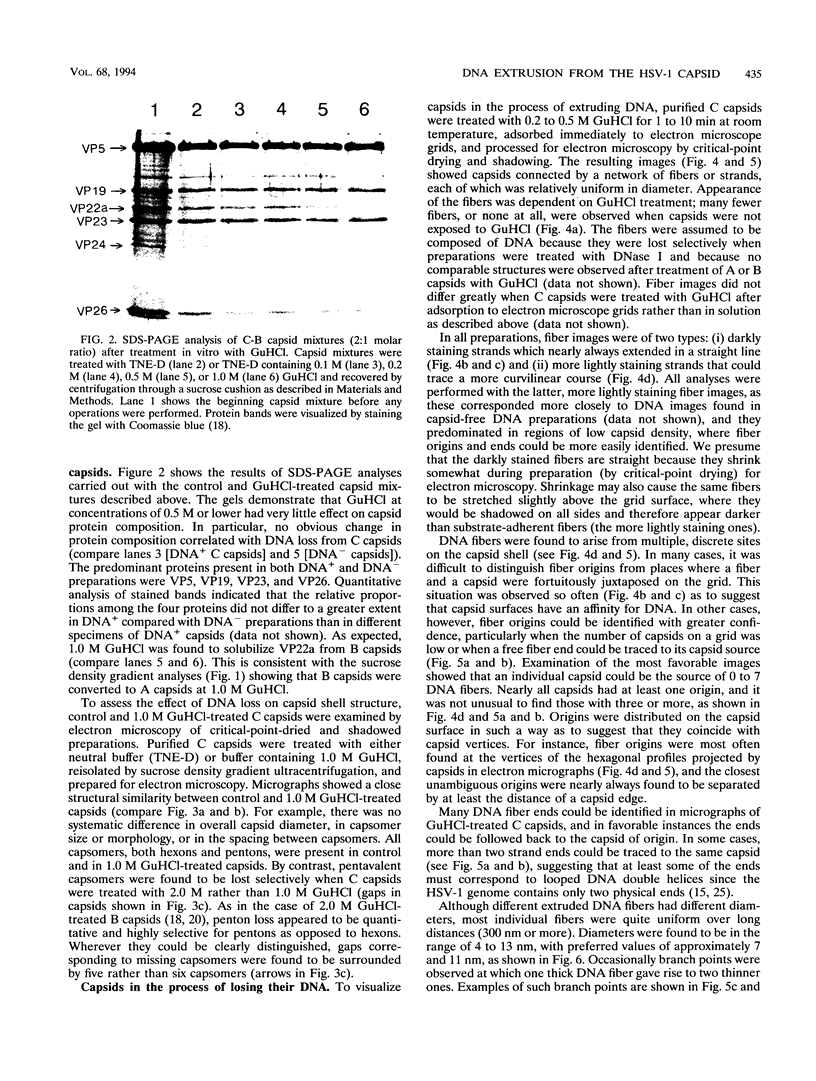
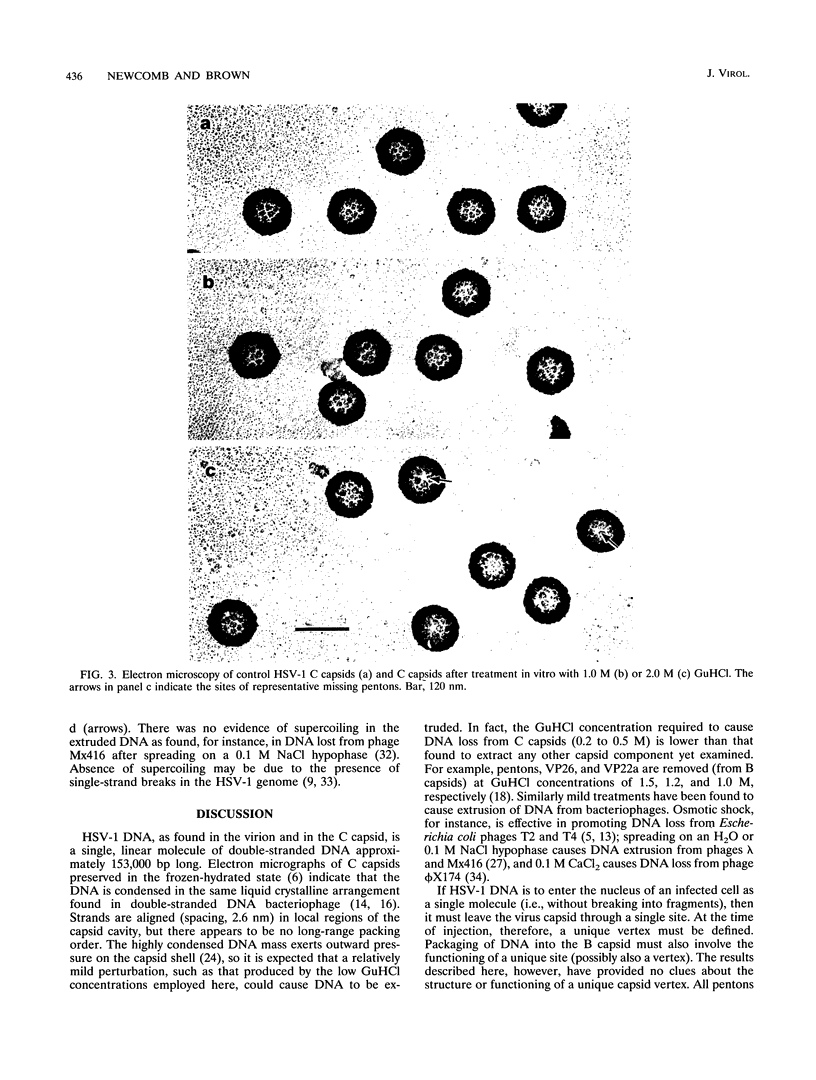
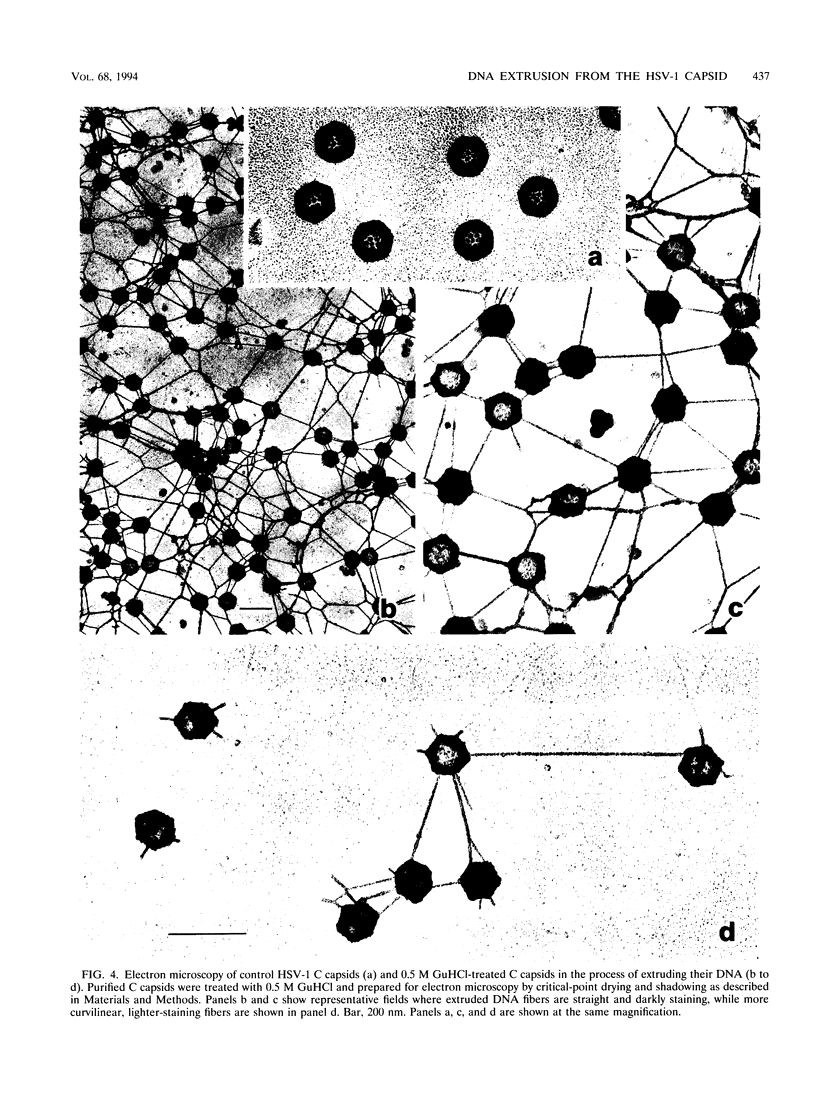
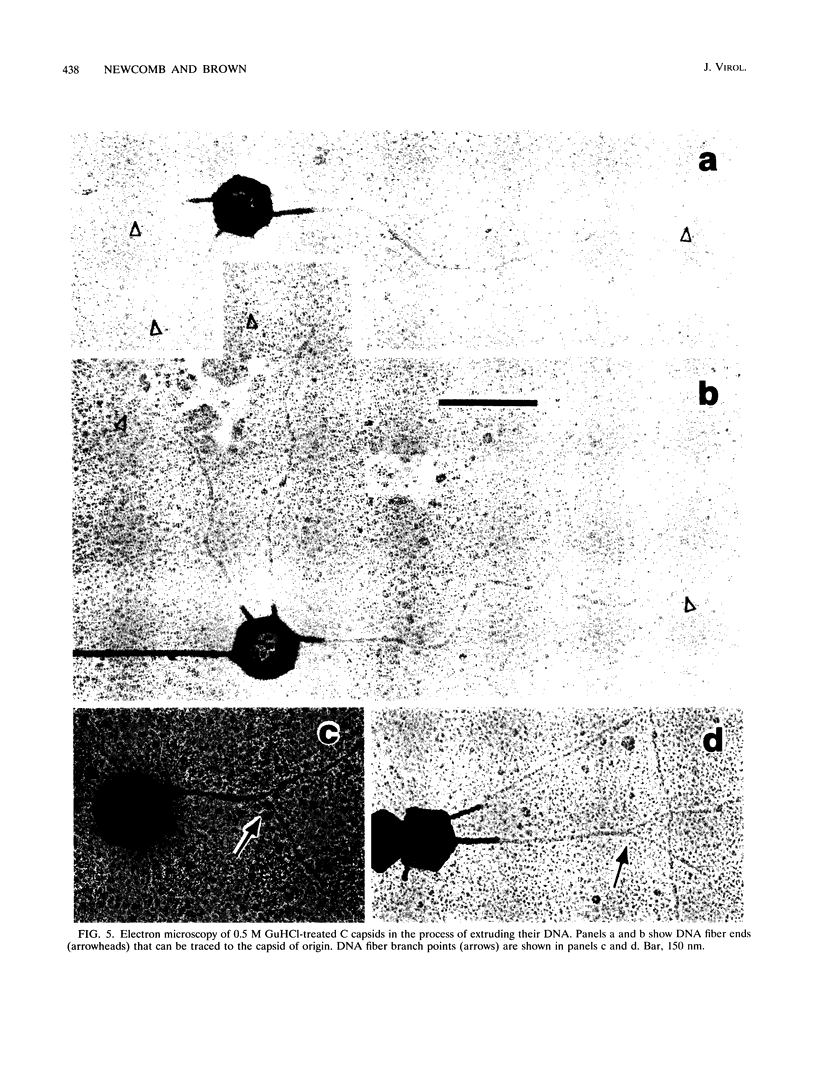
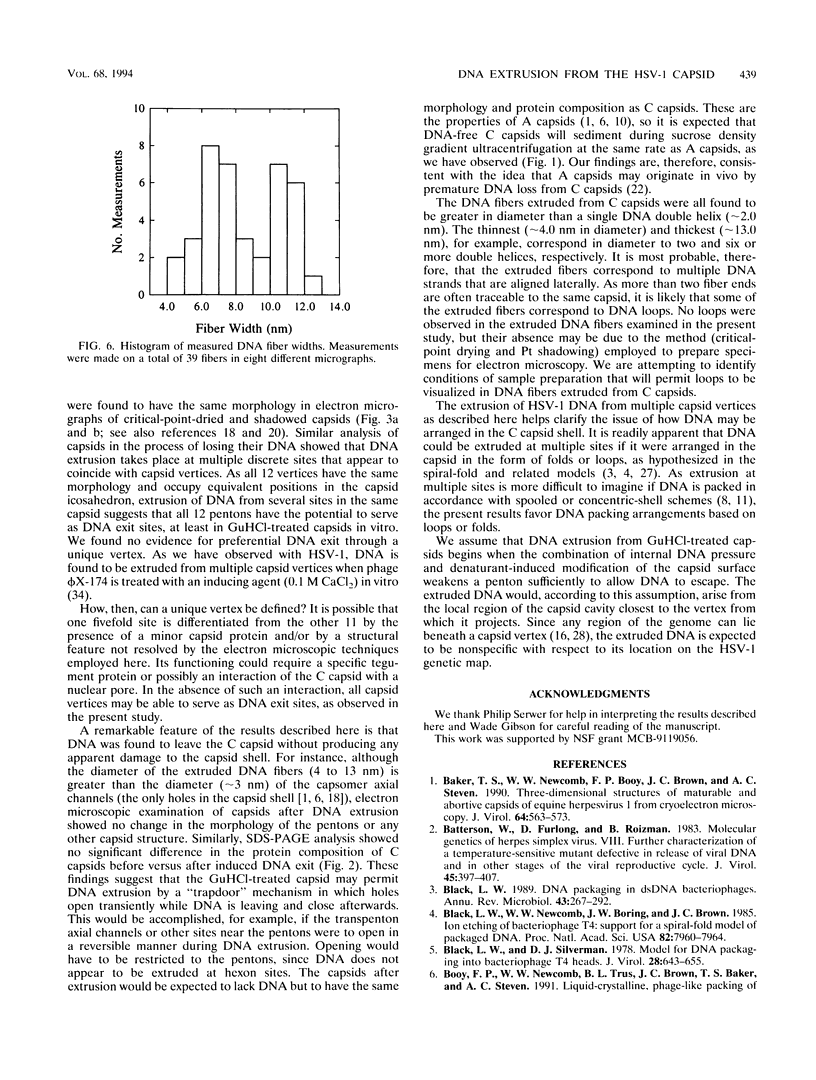
DNA-filled capsids (C capsids) of herpes simplex virus type 1 were treated in vitro with guanidine-HCl (GuHCl) and analyzed for DNA loss by sucrose density gradient ultracentrifugation and electron microscopy. DNA was found to be lost quantitatively from virtually all capsids treated with GuHCl at concentrations of 0.5 M or higher, while 0.1 M GuHCl had little or no effect. DNA removal from 0.5 M GuHCl-treated capsids was effected without significant change in the capsid protein composition, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, or in its structure, as judged by electron microscopy. Electron microscopic examination of capsids in the process of emptying showed that DNA was extruded from multiple, discrete sites which appeared to coincide with capsid vertices. DNA exited the capsid in the form of thick strands or fibers that varied in diameter from approximately 4 to 13 nm with preferred diameters of 7 and 11 nm. The fibers most probably correspond to multiple, laterally aligned DNA segments, as their diameters are nearly all greater than that of a single DNA double helix. The results suggest that GuHCl treatment promotes an alteration in the capsid pentons which allows DNA to escape locally. Hexons must be more resistant to this change, since DNA loss appears to be restricted to the pentons. The ability of GuHCl to cause loss of DNA from C capsids with no accompanying change in capsid morphology or protein composition suggests that penton sites may open transiently to permit DNA exist and then return to their original state.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. S., Newcomb W. W., Booy F. P., Brown J. C., Steven A. C. Three-dimensional structures of maturable and abortive capsids of equine herpesvirus 1 from cryoelectron microscopy. J Virol. 1990 Feb;64(2):563–573. doi: 10.1128/jvi.64.2.563-573.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterson W., Furlong D., Roizman B. Molecular genetics of herpes simplex virus. VIII. further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J Virol. 1983 Jan;45(1):397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black L. W. DNA packaging in dsDNA bacteriophages. Annu Rev Microbiol. 1989;43:267–292. doi: 10.1146/annurev.mi.43.100189.001411. [DOI] [PubMed] [Google Scholar]
- Black L. W., Newcomb W. W., Boring J. W., Brown J. C. Ion etching bacteriophage T4: support for a spiral-fold model of packaged DNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7960–7964. doi: 10.1073/pnas.82.23.7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black L. W., Silverman D. J. Model for DNA packaging into bacteriophage T4 heads. J Virol. 1978 Nov;28(2):643–655. doi: 10.1128/jvi.28.2.643-655.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Separation of the herpesvirus deoxyribonucleic acid duplex into unique fragments and intact strand on sedimentation in alkaline gradients. J Virol. 1972 Oct;10(4):565–572. doi: 10.1128/jvi.10.4.565-572.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C. Packaging of DNA into bacteriophage heads: a model. J Mol Biol. 1983 Dec 25;171(4):577–580. doi: 10.1016/0022-2836(83)90045-1. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- Lepault J., Dubochet J., Baschong W., Kellenberger E. Organization of double-stranded DNA in bacteriophages: a study by cryo-electron microscopy of vitrified samples. EMBO J. 1987 May;6(5):1507–1512. doi: 10.1002/j.1460-2075.1987.tb02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J. The genomes of the human herpesviruses: contents, relationships, and evolution. Annu Rev Microbiol. 1989;43:235–265. doi: 10.1146/annurev.mi.43.100189.001315. [DOI] [PubMed] [Google Scholar]
- Mendelson E. C., Newcomb W. W., Brown J. C. Ar+ plasma-induced damage to DNA in bacteriophage lambda: implications for the arrangement of DNA in the phage head. J Virol. 1992 Apr;66(4):2226–2231. doi: 10.1128/jvi.66.4.2226-2231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C., Booy F. P., Steven A. C. Nucleocapsid mass and capsomer protein stoichiometry in equine herpesvirus 1: scanning transmission electron microscopic study. J Virol. 1989 Sep;63(9):3777–3783. doi: 10.1128/jvi.63.9.3777-3783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J Virol. 1991 Feb;65(2):613–620. doi: 10.1128/jvi.65.2.613-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Use of Ar+ plasma etching to localize structural proteins in the capsid of herpes simplex virus type 1. J Virol. 1989 Nov;63(11):4697–4702. doi: 10.1128/jvi.63.11.4697-4702.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Trus B. L., Booy F. P., Steven A. C., Wall J. S., Brown J. C. Structure of the herpes simplex virus capsid. Molecular composition of the pentons and the triplexes. J Mol Biol. 1993 Jul 20;232(2):499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Cohen J. C., Kemp M. C., Randall C. C., O'Callaghan D. J. Characterization of three species of nucleocapsids of equine herpesvirus type-1 (EHV-1). Virology. 1975 Mar;64(1):187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Coates J. A., Rixon F. J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983 Mar;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Lee B., Parsegian V. A. Measurement of the repulsive force between polyelectrolyte molecules in ionic solution: hydration forces between parallel DNA double helices. Proc Natl Acad Sci U S A. 1984 May;81(9):2621–2625. doi: 10.1073/pnas.81.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag J. D., Prasad B. V., Rixon F. J., Chiu W. Three-dimensional structure of the HSV1 nucleocapsid. Cell. 1989 Feb 24;56(4):651–660. doi: 10.1016/0092-8674(89)90587-4. [DOI] [PubMed] [Google Scholar]
- Serwer P. Arrangement of double-stranded DNA packaged in bacteriophage capsids. An alternative model. J Mol Biol. 1986 Aug 5;190(3):509–512. doi: 10.1016/0022-2836(86)90019-7. [DOI] [PubMed] [Google Scholar]
- Serwer P., Hayes S. J., Watson R. H. Conformation of DNA packaged in bacteriophage T7. Analysis by use of ultraviolet light-induced DNA-capsid cross-linking. J Mol Biol. 1992 Feb 20;223(4):999–1011. doi: 10.1016/0022-2836(92)90258-l. [DOI] [PubMed] [Google Scholar]
- Sherman G., Bachenheimer S. L. Characterization of intranuclear capsids made by ts morphogenic mutants of HSV-1. Virology. 1988 Apr;163(2):471–480. doi: 10.1016/0042-6822(88)90288-7. [DOI] [PubMed] [Google Scholar]
- Tognon M., Furlong D., Conley A. J., Roizman B. Molecular genetics of herpes simplex virus. V. Characterization of a mutant defective in ability to form plaques at low temperatures and in a viral fraction which prevents accumulation of coreless capsids at nuclear pores late in infection. J Virol. 1981 Dec;40(3):870–880. doi: 10.1128/jvi.40.3.870-880.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus B. L., Newcomb W. W., Booy F. P., Brown J. C., Steven A. C. Distinct monoclonal antibodies separately label the hexons or the pentons of herpes simplex virus capsid. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11508–11512. doi: 10.1073/pnas.89.23.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virrankoski-Castrodeza V., Parish J. H. Evidence for supercoiling in the DNA of bacteriophage heads. Arch Microbiol. 1980 Jul;126(3):277–283. doi: 10.1007/BF00409932. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M. The synthesis and substructure of herpesvirus DNA: the distribution of alkali-labile single strand interruptions in HSV-1 DNA. J Gen Virol. 1973 Dec;21(3):453–467. doi: 10.1099/0022-1317-21-3-453. [DOI] [PubMed] [Google Scholar]
- Yazaki K. Electron microscopic studies of bacteriophage phi X174 intact and "eclipsing' particles, and the genome by the staining, and shadowing method. J Virol Methods. 1981 Feb;2(3):159–167. doi: 10.1016/0166-0934(81)90034-3. [DOI] [PubMed] [Google Scholar]