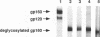Abstract
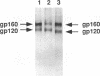
The human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein has been shown to be extensively modified by N-linked glycosylation; however, the presence of O-linked carbohydrates on the glycoprotein has not been firmly established. We have found that enzymatic deglycosylation of the HIV-1 envelope glycoprotein with neuraminidase and O-glycosidase results in a decrease in the apparent molecular weight of the envelope glycoprotein. This result was observed in both vaccinia virus recombinant-derived envelope glycoproteins and glycoproteins derived from the IIIB, SG3, and HXB2, strains of HIV-1. The decrease in molecular weight was also observed when the envelope glycoprotein had been deglycosylated with N-glycanase F after treatment with neuraminidase and O-glycosidase, indicating that the decrease in apparent molecular weight was not attributable to the removal of N-linked carbohydrate. Treatment with neuraminidase, O-glycosidase, and N-glycanase F was found to be necessary to remove all radiolabel from [3H]glucosamine-labelled envelope glycoprotein, a result seen for both recombinant and HIV-1-derived envelope glycoprotein. [3H]glucosamine-labelled carbohydrates liberated by O-glycosidase treatment were separated by paper chromatography and were found to be of a size consistent with O-linked oligosaccharides. We, therefore, conclude that the HIV-1 envelope glycoprotein is modified by the addition of O-linked carbohydrates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. S., Coligan J. E., Barin F., McLane M. F., Sodroski J. G., Rosen C. A., Haseltine W. A., Lee T. H., Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985 May 31;228(4703):1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- Bernstein H. B., Compans R. W. Sulfation of the human immunodeficiency virus envelope glycoprotein. J Virol. 1992 Dec;66(12):6953–6959. doi: 10.1128/jvi.66.12.6953-6959.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Merkle R. K., Stults N. L. Separation and analysis of glycoprotein oligosaccharides. Methods Cell Biol. 1989;32:141–183. doi: 10.1016/s0091-679x(08)61170-x. [DOI] [PubMed] [Google Scholar]
- Dewar R. L., Vasudevachari M. B., Natarajan V., Salzman N. P. Biosynthesis and processing of human immunodeficiency virus type 1 envelope glycoproteins: effects of monensin on glycosylation and transport. J Virol. 1989 Jun;63(6):2452–2456. doi: 10.1128/jvi.63.6.2452-2456.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Doms R. W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991 Apr;65(4):2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H., Will C., Schikore M., Slenczka W., Klenk H. D. Glycosylation and oligomerization of the spike protein of Marburg virus. Virology. 1991 May;182(1):353–356. doi: 10.1016/0042-6822(91)90680-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 1987 Jul 31;50(3):327–328. doi: 10.1016/0092-8674(87)90485-5. [DOI] [PubMed] [Google Scholar]
- Geyer H., Holschbach C., Hunsmann G., Schneider J. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J Biol Chem. 1988 Aug 25;263(24):11760–11767. [PubMed] [Google Scholar]
- Geyer R., Dabrowski J., Dabrowski U., Linder D., Schlüter M., Schott H. H., Stirm S. Oligosaccharides at individual glycosylation sites in glycoprotein 71 of Friend murine leukemia virus. Eur J Biochem. 1990 Jan 12;187(1):95–110. doi: 10.1111/j.1432-1033.1990.tb15281.x. [DOI] [PubMed] [Google Scholar]
- Ghosh S. K., Fultz P. N., Keddie E., Saag M. S., Sharp P. M., Hahn B. H., Shaw G. M. A molecular clone of HIV-1 tropic and cytopathic for human and chimpanzee lymphocytes. Virology. 1993 Jun;194(2):858–864. doi: 10.1006/viro.1993.1331. [DOI] [PubMed] [Google Scholar]
- Hammar L., Eriksson S., Morein B. Human immunodeficiency virus glycoproteins: lectin binding properties. AIDS Res Hum Retroviruses. 1989 Oct;5(5):495–506. doi: 10.1089/aid.1989.5.495. [DOI] [PubMed] [Google Scholar]
- Hansen J. E., Clausen H., Nielsen C., Teglbjaerg L. S., Hansen L. L., Nielsen C. M., Dabelsteen E., Mathiesen L., Hakomori S. I., Nielsen J. O. Inhibition of human immunodeficiency virus (HIV) infection in vitro by anticarbohydrate monoclonal antibodies: peripheral glycosylation of HIV envelope glycoprotein gp120 may be a target for virus neutralization. J Virol. 1990 Jun;64(6):2833–2840. doi: 10.1128/jvi.64.6.2833-2840.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. R., Srinivas R. V., Stephens E. B., Compans R. W. Effects of deletion of the cytoplasmic domain upon surface expression and membrane stability of a viral envelope glycoprotein. J Biol Chem. 1987 Nov 25;262(33):16116–16121. [PubMed] [Google Scholar]
- Kozarsky K., Penman M., Basiripour L., Haseltine W., Sodroski J., Krieger M. Glycosylation and processing of the human immunodeficiency virus type 1 envelope protein. J Acquir Immune Defic Syndr. 1989;2(2):163–169. [PubMed] [Google Scholar]
- Krieger M., Reddy P., Kozarsky K., Kingsley D., Hobbie L., Penman M. Analysis of the synthesis, intracellular sorting, and function of glycoproteins using a mammalian cell mutant with reversible glycosylation defects. Methods Cell Biol. 1989;32:57–84. doi: 10.1016/s0091-679x(08)61167-x. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Groopman J. E., Fennie C. W., Benz P. M., Capon D. J., Dowbenko D. J., Nakamura G. R., Nunes W. M., Renz M. E., Berman P. W. Neutralization of the AIDS retrovirus by antibodies to a recombinant envelope glycoprotein. Science. 1986 Jul 11;233(4760):209–212. doi: 10.1126/science.3014647. [DOI] [PubMed] [Google Scholar]
- Lee W. R., Yu X. F., Syu W. J., Essex M., Lee T. H. Mutational analysis of conserved N-linked glycosylation sites of human immunodeficiency virus type 1 gp41. J Virol. 1992 Mar;66(3):1799–1803. doi: 10.1128/jvi.66.3.1799-1803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C. K., Spellman M. W., Riddle L., Harris R. J., Thomas J. N., Gregory T. J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990 Jun 25;265(18):10373–10382. [PubMed] [Google Scholar]
- Matthews T. J., Weinhold K. J., Lyerly H. K., Langlois A. J., Wigzell H., Bolognesi D. P. Interaction between the human T-cell lymphotropic virus type IIIB envelope glycoprotein gp120 and the surface antigen CD4: role of carbohydrate in binding and cell fusion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5424–5428. doi: 10.1073/pnas.84.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Nicholson J. K., Cross G. D., Cort S. P., Kennedy M. S., Mawle A. C. Binding of the human retrovirus HTLV-III/LAV/ARV/HIV to the CD4 (T4) molecule: conformation dependence, epitope mapping, antibody inhibition, and potential for idiotypic mimicry. J Immunol. 1986 Nov 1;137(9):2937–2944. [PubMed] [Google Scholar]
- Merkle R. K., Helland D. E., Welles J. L., Shilatifard A., Haseltine W. A., Cummings R. D. gp160 of HIV-I synthesized by persistently infected Molt-3 cells is terminally glycosylated: evidence that cleavage of gp160 occurs subsequent to oligosaccharide processing. Arch Biochem Biophys. 1991 Oct;290(1):248–257. doi: 10.1016/0003-9861(91)90616-q. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Matthews T. J., Kato M., Hamako J., Titani K., Solomon J., Feizi T. Diversity of oligosaccharide structures on the envelope glycoprotein gp 120 of human immunodeficiency virus 1 from the lymphoblastoid cell line H9. Presence of complex-type oligosaccharides with bisecting N-acetylglucosamine residues. J Biol Chem. 1990 May 25;265(15):8519–8524. [PubMed] [Google Scholar]
- Moore B. R., Free S. J. Protein modification and its biological role. Int J Biochem. 1985;17(3):283–289. doi: 10.1016/0020-711x(85)90202-2. [DOI] [PubMed] [Google Scholar]
- Niemann H., Geyer R., Klenk H. D., Linder D., Stirm S., Wirth M. The carbohydrates of mouse hepatitis virus (MHV) A59: structures of the O-glycosidically linked oligosaccharides of glycoprotein E1. EMBO J. 1984 Mar;3(3):665–670. doi: 10.1002/j.1460-2075.1984.tb01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbaugh J., Rudensey L. M. Alterations in potential sites for glycosylation predominate during evolution of the simian immunodeficiency virus envelope gene in macaques. J Virol. 1992 Oct;66(10):5937–5948. doi: 10.1128/jvi.66.10.5937-5948.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Mumbauer S., Hoke G. M., Takatsuki A., Sarngadharan M. G. Brefeldin A inhibits the processing and secretion of envelope glycoproteins of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1991 Aug;7(8):707–712. doi: 10.1089/aid.1991.7.707. [DOI] [PubMed] [Google Scholar]
- Paulson J. C. Glycoproteins: what are the sugar chains for? Trends Biochem Sci. 1989 Jul;14(7):272–276. doi: 10.1016/0968-0004(89)90062-5. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J. Biochemical characterization of cell-associated and extracellular products of the Friend spleen focus-forming virus env gene. Virology. 1989 Nov;173(1):136–143. doi: 10.1016/0042-6822(89)90229-8. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J. O-linked glycosylation of retroviral envelope gene products. J Virol. 1988 Mar;62(3):1016–1021. doi: 10.1128/jvi.62.3.1016-1021.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Safai B., Oroszlan S., Arthur L. O., Gonda M. A., Gallo R. C., Fischinger P. J. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science. 1985 May 3;228(4699):593–595. doi: 10.1126/science.2984774. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kaaden O., Copeland T. D., Oroszlan S., Hunsmann G. Shedding and interspecies type sero-reactivity of the envelope glycopolypeptide gp120 of the human immunodeficiency virus. J Gen Virol. 1986 Nov;67(Pt 11):2533–2538. doi: 10.1099/0022-1317-67-11-2533. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Engleman E. G. Intracellular processing of the gp160 HIV-1 envelope precursor. Endoproteolytic cleavage occurs in a cis or medial compartment of the Golgi complex. J Biol Chem. 1990 Feb 15;265(5):2640–2649. [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985 Sep 27;229(4720):1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Bonifacino J. S., Potts B. J., Martin M. A., Klausner R. D. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]