Abstract
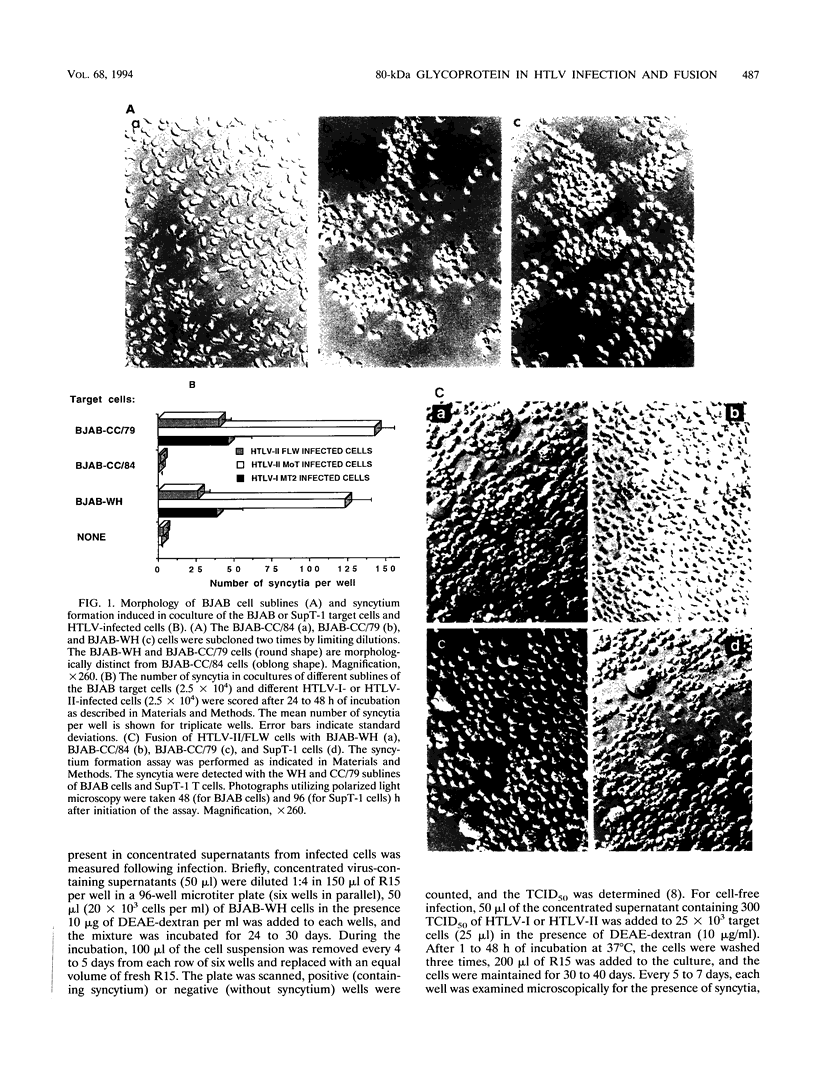
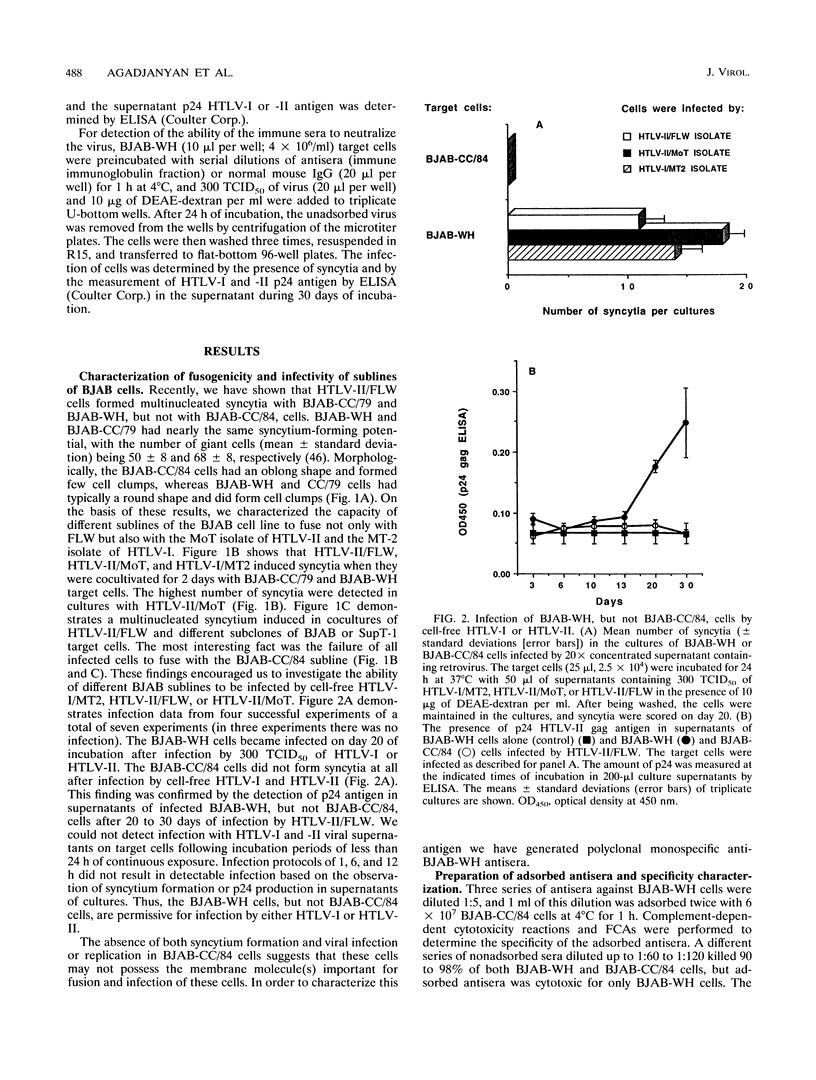
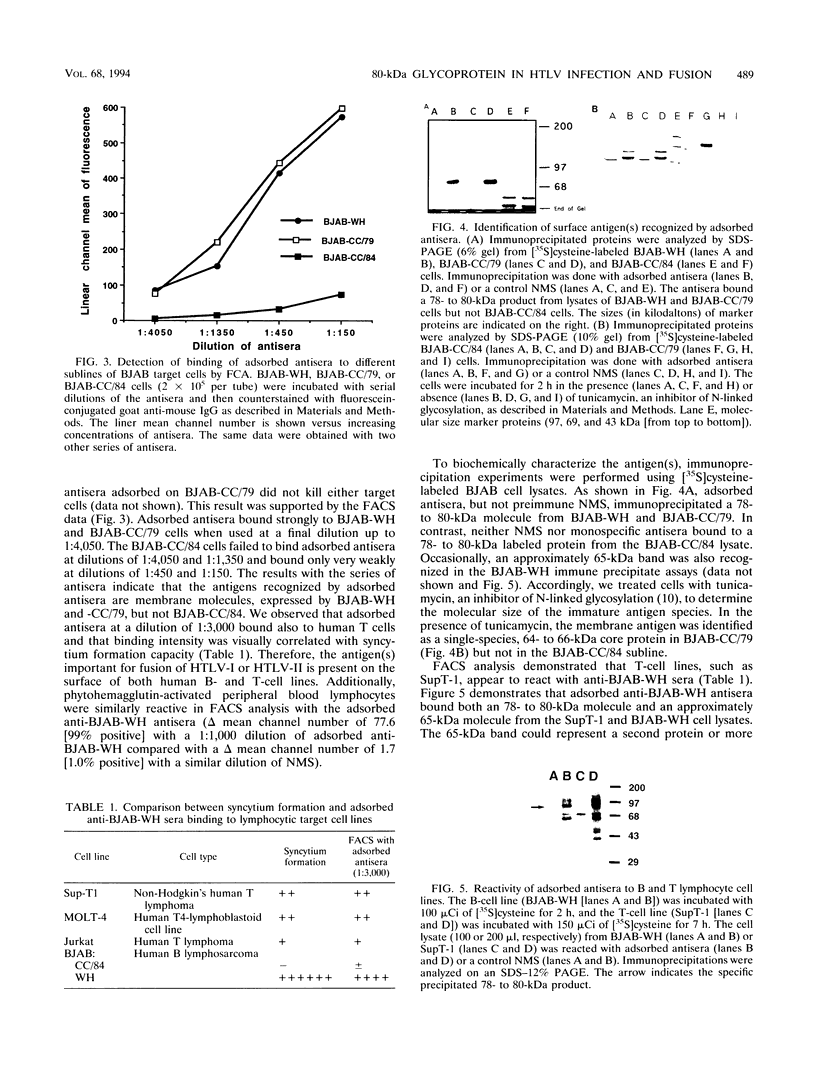
Human T-cell leukemia virus type I and type II (HTLV-I and HTLV-II, respectively) infect certain sublines of the BJAB human B-cell line. We observed that the WH subline, but not the CC/84 subline, of BJAB cells were infectible by cell-free HTLV-I or HTLV-II and formed syncytia with cells infected by these retroviruses. This suggests that the BJAB-CC/84 cells possibly lack a membrane molecule(s) important for syncytium formation and infectibility. In order to identify this antigen, we generated polyclonal anti-BJAB-WH antisera which were adsorbed on BJAB-CC/84 cells. The adsorbed antisera bound only BJAB-WH and BJAB-CC/79 cells as demonstrated by complement-dependent cytotoxicity and flow cytometric assays. Furthermore, this adsorbed antisera bound several human T-cell clones, including SupT-1, as determined by flow cytometric assays. The adsorbed antiserum was monospecific as it immunoprecipitated only one 78- to 80-kDa protein from lysates of metabolically labeled BJAB-WH, BJAB-CC/79, and SupT-1, but not BJAB-CC/84, cells. The monospecific antisera detected a glycoprotein composed of a 64- to 66-kDa core protein containing tunicamycin-sensitive N-linked oligosaccharides. This membrane glycoprotein appears to be involved in HTLV-I- and HTLV-II-induced fusion and infection, as the monospecific antisera were capable of inhibiting both of these processes. The monospecific antisera diluted 1:50 and 1:90 inhibited 85 to 90% of syncytium formation induced in BJAB-WH, BJAB-CC/79, and SupT-1 cells cultured with HTLV-I- or HTLV-II-infected MT2, MoT, or FLW human T- or B-cell lines. At the same dilution, antisera inhibited 70 to 80% of infection of BJAB-WH cells by cell-free HTLV-I or HTLV-II. Thus, these studies indicate a role for a 78- to 80-kDa glycoprotein in HTLV-I or HTLV-II infection and syncytium formation.
Full text
PDF

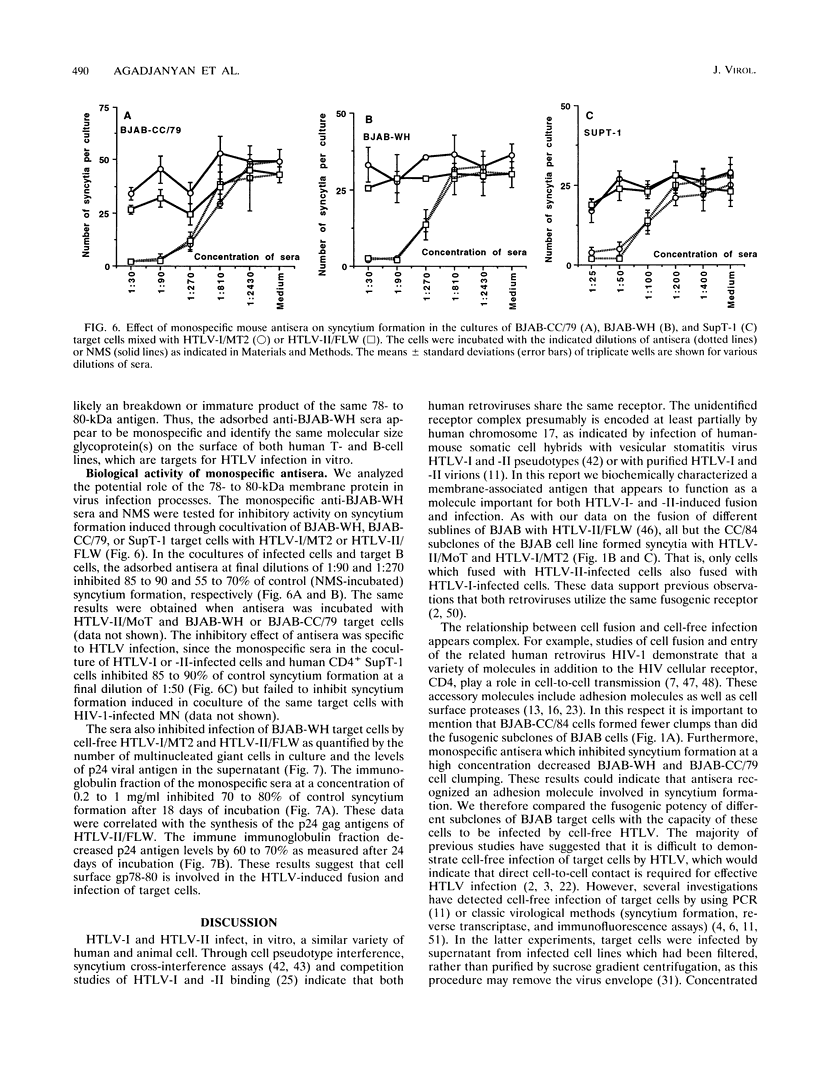
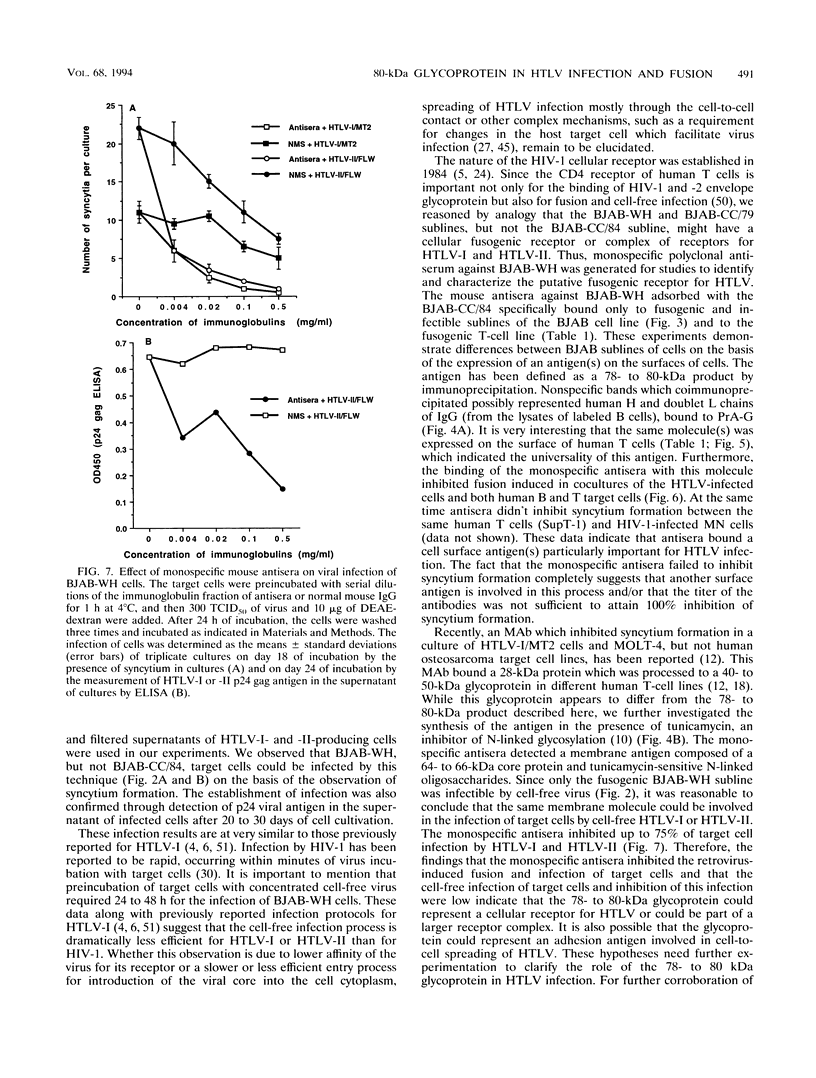


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner W. A., Kalyanaraman V. S., Robert-Guroff M., Lister T. A., Galton D. A., Sarin P. S., Crawford M. H., Catovsky D., Greaves M., Gallo R. C. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int J Cancer. 1982 Sep 15;30(3):257–264. doi: 10.1002/ijc.2910300302. [DOI] [PubMed] [Google Scholar]
- Clapham P., Nagy K., Cheingsong-Popov R., Exley M., Weiss R. A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983 Dec 9;222(4628):1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dragic T., Charneau P., Clavel F., Alizon M. Complementation of murine cells for human immunodeficiency virus envelope/CD4-mediated fusion in human/murine heterokaryons. J Virol. 1992 Aug;66(8):4794–4802. doi: 10.1128/jvi.66.8.4794-4802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G. D., Davey F. R., Kirshner J. J., Sninsky J. J., Kwok S., Slamon D. J., Kalish R., Poiesz B. J. A polyclonal CD4+ and CD8+ lymphocytosis in a patient doubly infected with HTLV-I and HIV-1: a clinical and molecular analysis. Am J Hematol. 1989 Mar;30(3):128–139. doi: 10.1002/ajh.2830300304. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Fan N., Gavalchin J., Paul B., Wells K. H., Lane M. J., Poiesz B. J. Infection of peripheral blood mononuclear cells and cell lines by cell-free human T-cell lymphoma/leukemia virus type I. J Clin Microbiol. 1992 Apr;30(4):905–910. doi: 10.1128/jcm.30.4.905-910.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudome K., Furuse M., Imai T., Nishimura M., Takagi S., Hinuma Y., Yoshie O. Identification of membrane antigen C33 recognized by monoclonal antibodies inhibitory to human T-cell leukemia virus type 1 (HTLV-1)-induced syncytium formation: altered glycosylation of C33 antigen in HTLV-1-positive T cells. J Virol. 1992 Mar;66(3):1394–1401. doi: 10.1128/jvi.66.3.1394-1401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber M. F., Webb D. S., Gerrard T. L., Mostowski H. S., Vujcic L., Golding H. Re-evaluation of the involvement of the adhesion molecules ICAM-1/LFA-1 in syncytia formation of HIV-1-infected subclones of a CEM T-cell leukemic line. AIDS Res Hum Retroviruses. 1991 Jan;7(1):45–53. doi: 10.1089/aid.1991.7.45. [DOI] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E., Orentas R. J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989 Jun 2;244(4908):1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- Hoshino H., Shimoyama M., Miwa M., Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7337–7341. doi: 10.1073/pnas.80.23.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T., Fukudome K., Takagi S., Nagira M., Furuse M., Fukuhara N., Nishimura M., Hinuma Y., Yoshie O. C33 antigen recognized by monoclonal antibodies inhibitory to human T cell leukemia virus type 1-induced syncytium formation is a member of a new family of transmembrane proteins including CD9, CD37, CD53, and CD63. J Immunol. 1992 Nov 1;149(9):2879–2886. [PubMed] [Google Scholar]
- Kalyanaraman V. S., Narayanan R., Feorino P., Ramsey R. B., Palmer E. L., Chorba T., McDougal S., Getchell J. P., Holloway B., Harrison A. K. Isolation and characterization of a human T cell leukemia virus type II from a hemophilia-A patient with pancytopenia. EMBO J. 1985 Jun;4(6):1455–1460. doi: 10.1002/j.1460-2075.1985.tb03802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Kaplan M. H., Hall W. W., Susin M., Pahwa S., Salahuddin S. Z., Heilman C., Fetten J., Coronesi M., Farber B. F., Smith S. Syndrome of severe skin disease, eosinophilia, and dermatopathic lymphadenopathy in patients with HTLV-II complicating human immunodeficiency virus infection. Am J Med. 1991 Sep;91(3):300–309. doi: 10.1016/0002-9343(91)90132-h. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Krichbaum-Stenger K., Poiesz B. J., Keller P., Ehrlich G., Gavalchin J., Davis B. H., Moore J. L. Specific adsorption of HTLV-I to various target human and animal cells. Blood. 1987 Nov;70(5):1303–1311. [PubMed] [Google Scholar]
- Lee H., Swanson P., Shorty V. S., Zack J. A., Rosenblatt J. D., Chen I. S. High rate of HTLV-II infection in seropositive i.v. drug abusers in New Orleans. Science. 1989 Apr 28;244(4903):471–475. doi: 10.1126/science.2655084. [DOI] [PubMed] [Google Scholar]
- Levy D. N., Fernandes L. S., Williams W. V., Weiner D. B. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell. 1993 Feb 26;72(4):541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- Longo D. L., Gelmann E. P., Cossman J., Young R. A., Gallo R. C., O'Brien S. J., Matis L. A. Isolation of HTLV-transformed B-lymphocyte clone from a patient with HTLV-associated adult T-cell leukaemia. Nature. 1984 Aug 9;310(5977):505–506. doi: 10.1038/310505a0. [DOI] [PubMed] [Google Scholar]
- Loughran T. P., Jr, Coyle T., Sherman M. P., Starkebaum G., Ehrlich G. D., Ruscetti F. W., Poiesz B. J. Detection of human T-cell leukemia/lymphoma virus, type II, in a patient with large granular lymphocyte leukemia. Blood. 1992 Sep 1;80(5):1116–1119. [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- McGrath M., Witte O., Pincus T., Weissman I. L. Retrovirus purification: method that conserves envelope glycoprotein and maximizes infectivity. J Virol. 1978 Mar;25(3):923–927. doi: 10.1128/jvi.25.3.923-927.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy K., Clapham P., Cheingsong-Popov R., Weiss R. A. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients' sera. Int J Cancer. 1983 Sep 15;32(3):321–328. doi: 10.1002/ijc.2910320310. [DOI] [PubMed] [Google Scholar]
- Page J. B., Lai S. H., Chitwood D. D., Klimas N. G., Smith P. C., Fletcher M. A. HTLV-I/II seropositivity and death from AIDS among HIV-1 seropositive intravenous drug users. Lancet. 1990 Jun 16;335(8703):1439–1441. doi: 10.1016/0140-6736(90)91456-k. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Mier J. W., Woods A. M., Gallo R. C. T-cell lines established from human T-lymphocytic neoplasias by direct response to T-cell growth factor. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6815–6819. doi: 10.1073/pnas.77.11.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Reitz M. S., Kalyanaraman V. S., Gallo R. C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. Nature. 1981 Nov 19;294(5838):268–271. doi: 10.1038/294268a0. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Weiss S. H., Giron J. A., Jennings A. M., Ginzburg H. M., Margolis I. B., Blattner W. A., Gallo R. C. Prevalence of antibodies to HTLV-I, -II, and -III in intravenous drug abusers from an AIDS endemic region. JAMA. 1986 Jun 13;255(22):3133–3137. [PubMed] [Google Scholar]
- Rosenblatt J. D., Golde D. W., Wachsman W., Giorgi J. V., Jacobs A., Schmidt G. M., Quan S., Gasson J. C., Chen I. S. A second isolate of HTLV-II associated with atypical hairy-cell leukemia. N Engl J Med. 1986 Aug 7;315(6):372–377. doi: 10.1056/NEJM198608073150606. [DOI] [PubMed] [Google Scholar]
- Saxinger W., Blattner W. A., Levine P. H., Clark J., Biggar R., Hoh M., Moghissi J., Jacobs P., Wilson L., Jacobson R. Human T-cell leukemia virus (HTLV-I) antibodies in Africa. Science. 1984 Sep 28;225(4669):1473–1476. doi: 10.1126/science.6089348. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Weiss R. A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990 May;176(1):58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Williams B. P., Clapham P. R., Solomon E., Goodfellow P. N., Weiss R. A. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988 Dec 16;242(4885):1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- Tedder R. S., Shanson D. C., Jeffries D. J., Cheingsong-Popov R., Clapham P., Dalgleish A., Nagy K., Weiss R. A. Low prevalence in the UK of HTLV-I and HTLV-II infection in subjects with AIDS, with extended lymphadenopathy, and at risk of AIDS. Lancet. 1984 Jul 21;2(8395):125–128. doi: 10.1016/s0140-6736(84)91046-8. [DOI] [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Reynolds R. K., Luciw P. A., Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- Wang B., Agadjanyan M. G., Srikantan V., Ugen K. E., Hall W., Kaplan M. H., Dang K., Williams W. V., Weiner D. B. Molecular cloning, expression, and biological characterization of an HTLV-II envelope glycoprotein: HIV-1 expression is permissive for HTLV-II-induced cell fusion. AIDS Res Hum Retroviruses. 1993 Sep;9(9):849–860. doi: 10.1089/aid.1993.9.849. [DOI] [PubMed] [Google Scholar]
- Weiner D. B., Hubner K., Williams W. V., Greene M. I. Species tropism of HIV-1 infectivity of interspecific cell hybridomas implies non-CD4 structures are required for cell entry. Cancer Detect Prev. 1990;14(3):317–320. [PubMed] [Google Scholar]
- Weiner D. B., Liu J., Cohen J. A., Williams W. V., Greene M. I. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989 May 18;339(6221):230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P., Nagy K., Hoshino H. Envelope properties of human T-cell leukemia viruses. Curr Top Microbiol Immunol. 1985;115:235–246. doi: 10.1007/978-3-642-70113-9_15. [DOI] [PubMed] [Google Scholar]
- de Rossi A., Aldovini A., Franchini G., Mann D., Gallo R. C., Wong-Staal F. Clonal selection of T lymphocytes infected by cell-free human T-cell leukemia/lymphoma virus type I: parameters of virus integration and expression. Virology. 1985 Jun;143(2):640–645. doi: 10.1016/0042-6822(85)90405-2. [DOI] [PubMed] [Google Scholar]