Abstract
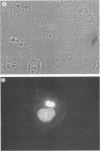
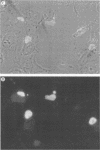
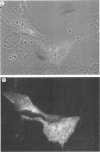
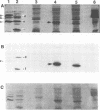
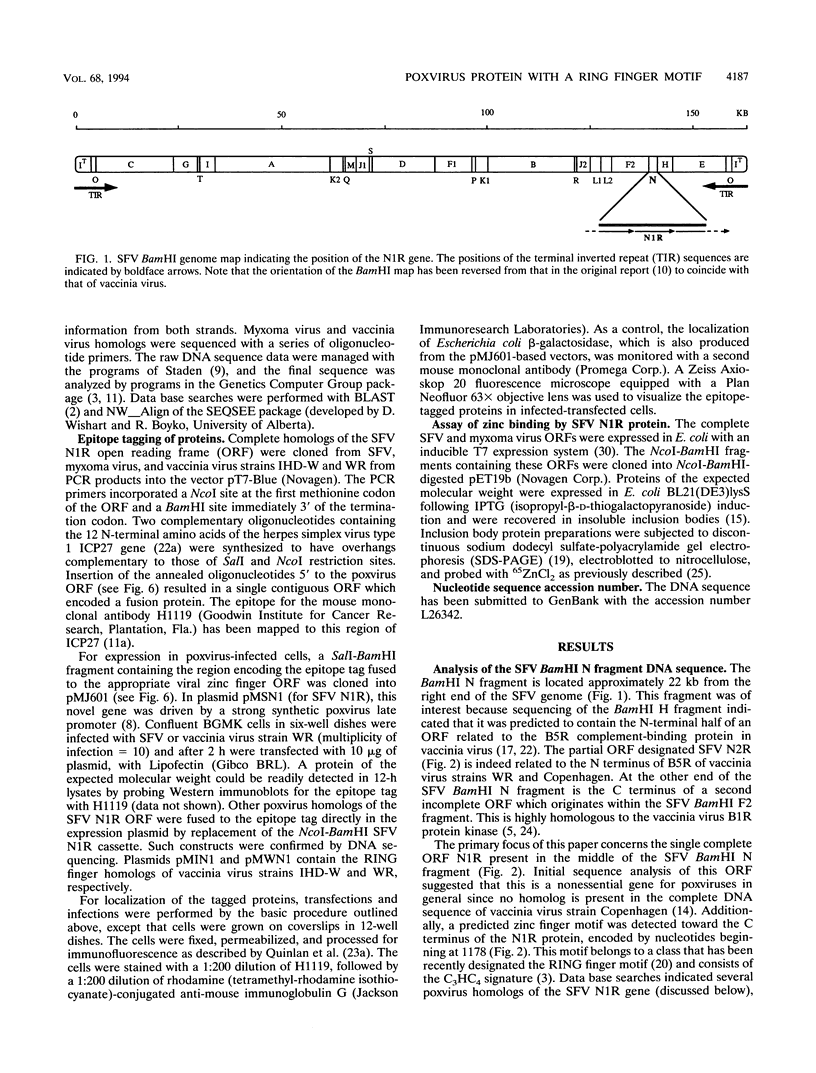
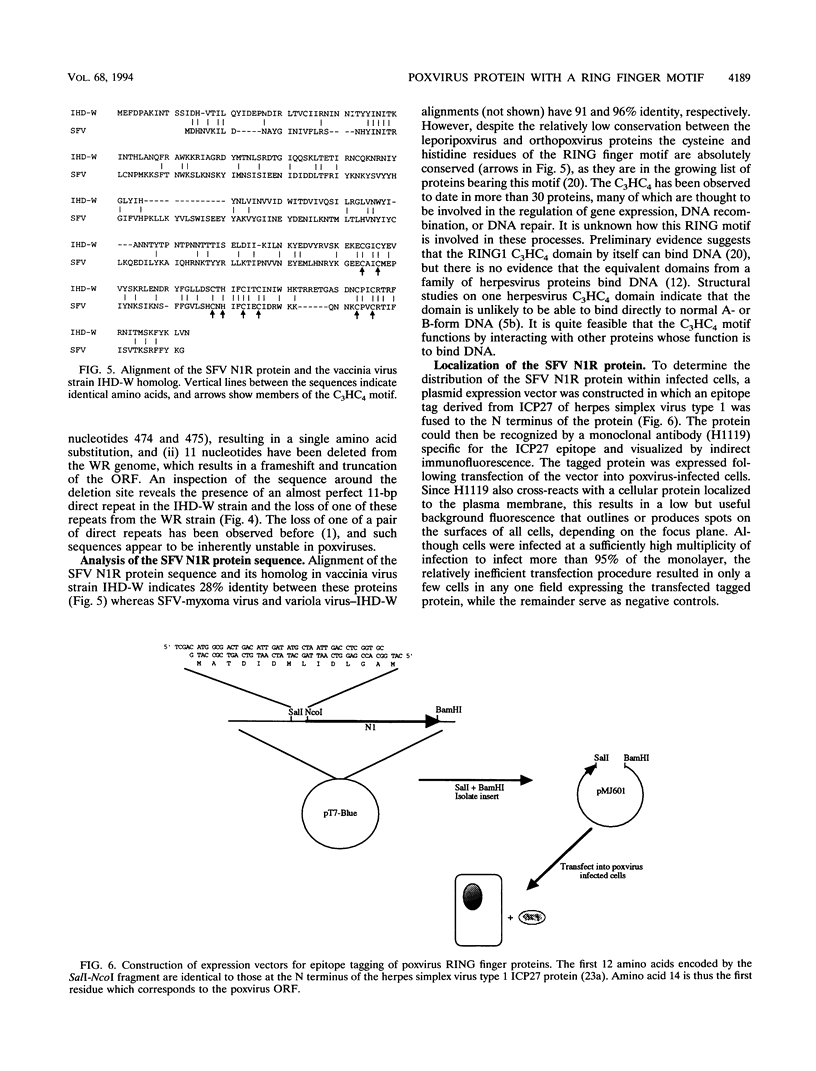
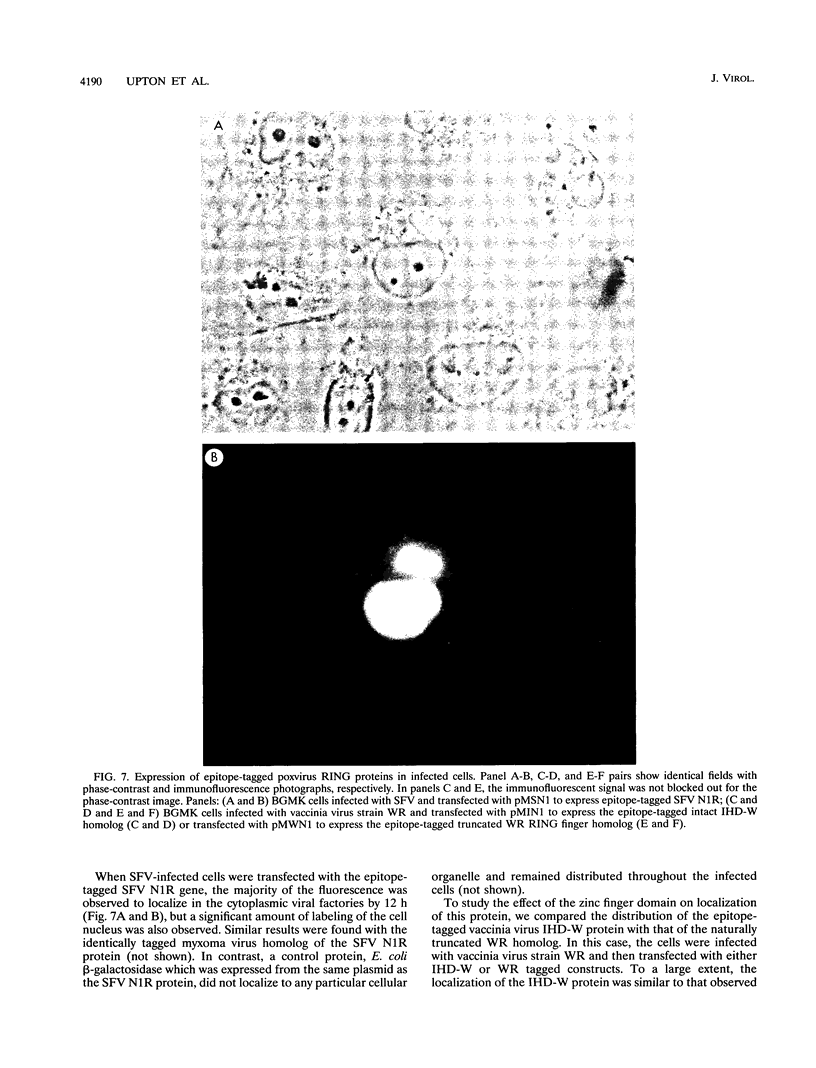
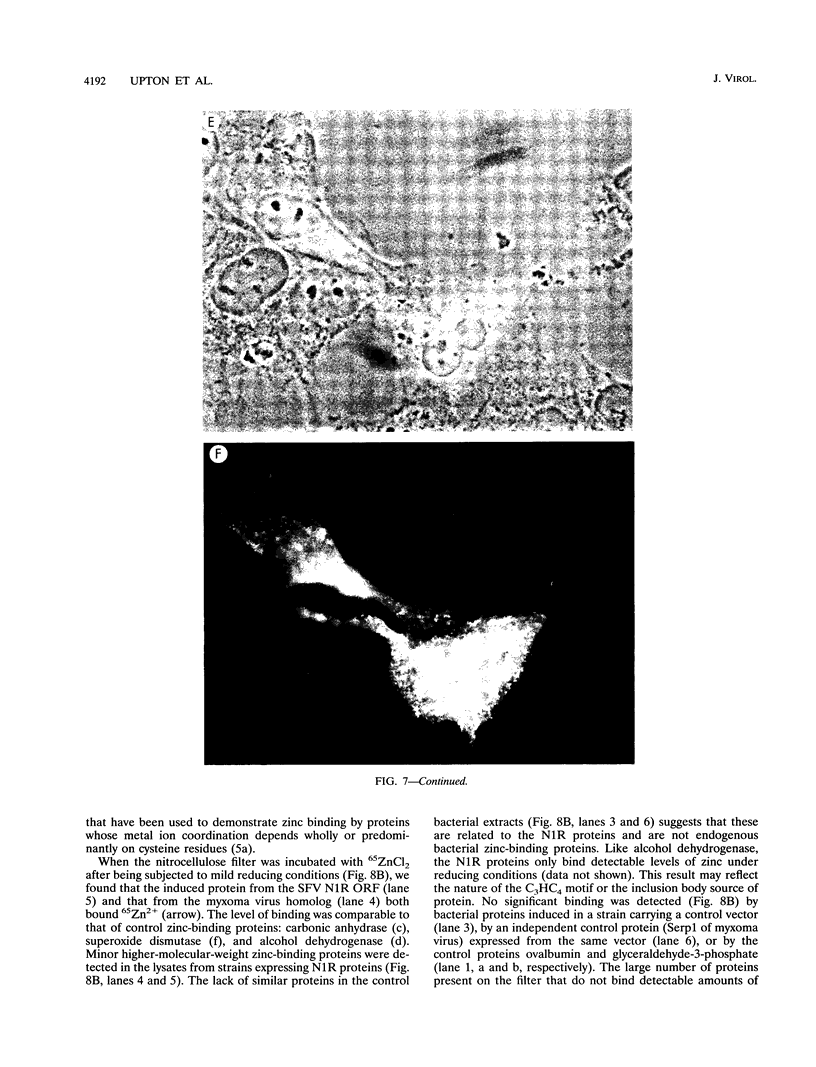
Shope fibroma virus (SFV) is a Leporipoxvirus closely related to the highly virulent myxoma virus. The DNA sequence of the BamHI N fragment of the SFV DNA genome was determined, and the single complete open reading frame (N1R) was characterized. The protein encoded by the N1R gene was found to contain a C3HC4 RING finger motif at the C terminus. This C3HC4 motif is the hallmark of a growing family of proteins, many of which are involved in regulation of gene expression, DNA repair, or DNA recombination. Complete homologs of the SFV N1R gene were also detected in variola virus, myxoma virus, and vaccinia virus strain IHD-W. In contrast, the gene is completely absent from vaccinia virus strain Copenhagen, and in vaccinia virus strain WR, the open reading frame is truncated prior to the zinc binding domain because of an 11-bp deletion, thus producing a frameshift and premature stop codon. Recombinant N1R protein from SFV was expressed in Escherichia coli and shown to bind zinc in a specific manner. Using fluorescence microscopy to visualize a peptide epitope tag (derived from ICP27 of herpes simplex virus) fused to the N terminus of the poxvirus proteins, we observed that the N1R protein of SFV and its homologs in myxoma virus and vaccinia virus IHD-W were localized primarily to the virus factories in the cytoplasm of infected cells and, to a lesser degree, the host cell nucleus. The truncated protein of vaccinia virus strain WR failed to localize in this manner but instead was observed throughout the cytoplasm.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguado B., Selmes I. P., Smith G. L. Nucleotide sequence of 21.8 kbp of variola major virus strain Harvey and comparison with vaccinia virus. J Gen Virol. 1992 Nov;73(Pt 11):2887–2902. doi: 10.1099/0022-1317-73-11-2887. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2241–2245. doi: 10.1093/nar/19.suppl.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldick C. J., Jr, Keck J. G., Moss B. Mutational analysis of the core, spacer, and initiator regions of vaccinia virus intermediate-class promoters. J Virol. 1992 Aug;66(8):4710–4719. doi: 10.1128/jvi.66.8.4710-4719.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banham A. H., Smith G. L. Vaccinia virus gene B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology. 1992 Dec;191(2):803–812. doi: 10.1016/0042-6822(92)90256-o. [DOI] [PubMed] [Google Scholar]
- Barbosa M. S., Lowy D. R., Schiller J. T. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol. 1989 Mar;63(3):1404–1407. doi: 10.1128/jvi.63.3.1404-1407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Palumbo G. J. Poxvirus pathogenesis. Microbiol Rev. 1991 Mar;55(1):80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Moss B. New vaccinia virus recombination plasmids incorporating a synthetic late promoter for high level expression of foreign proteins. Nucleic Acids Res. 1990 Jul 25;18(14):4285–4286. doi: 10.1093/nar/18.14.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Dear S., Staden R. A sequence assembly and editing program for efficient management of large projects. Nucleic Acids Res. 1991 Jul 25;19(14):3907–3911. doi: 10.1093/nar/19.14.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delange A. M., Macaulay C., Block W., Mueller T., McFadden G. Tumorigenic poxviruses: construction of the composite physical map of the Shope fibroma virus genome. J Virol. 1984 May;50(2):408–416. doi: 10.1128/jvi.50.2.408-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D., Barlow P., Milner A., Luisi B., Orr A., Hope G., Lyon D. A novel arrangement of zinc-binding residues and secondary structure in the C3HC4 motif of an alpha herpes virus protein family. J Mol Biol. 1993 Dec 20;234(4):1038–1047. doi: 10.1006/jmbi.1993.1657. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Keck J. G., Feigenbaum F., Moss B. Mutational analysis of a predicted zinc-binding motif in the 26-kilodalton protein encoded by the vaccinia virus A2L gene: correlation of zinc binding with late transcriptional transactivation activity. J Virol. 1993 Oct;67(10):5749–5753. doi: 10.1128/jvi.67.10.5749-5753.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Analysis of a large cluster of nonessential genes deleted from a vaccinia virus terminal transposition mutant. Virology. 1988 Dec;167(2):524–537. [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988 Sep 8;335(6186):176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lovering R., Hanson I. M., Borden K. L., Martin S., O'Reilly N. J., Evan G. I., Rahman D., Pappin D. J., Trowsdale J., Freemont P. S. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2112–2116. doi: 10.1073/pnas.90.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massung R. F., Esposito J. J., Liu L. I., Qi J., Utterback T. R., Knight J. C., Aubin L., Yuran T. E., Parsons J. M., Loparev V. N. Potential virulence determinants in terminal regions of variola smallpox virus genome. Nature. 1993 Dec 23;366(6457):748–751. doi: 10.1038/366748a0. [DOI] [PubMed] [Google Scholar]
- Massung R. F., McFadden G., Moyer R. W. Nucleotide sequence analysis of a unique near-terminal region of the tumorigenic poxvirus, Shope fibroma virus. J Gen Virol. 1992 Nov;73(Pt 11):2903–2911. doi: 10.1099/0022-1317-73-11-2903. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Rempel R. E., Anderson M. K., Evans E., Traktman P. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J Virol. 1990 Feb;64(2):574–583. doi: 10.1128/jvi.64.2.574-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff L. A., Nibert M. L., Fields B. N. Characterization of a zinc blotting technique: evidence that a retroviral gag protein binds zinc. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4195–4199. doi: 10.1073/pnas.85.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich T. G., Koonin E. V., Buller R. M. A poxvirus protein with a RING zinc finger motif is of crucial importance for virulence. Virology. 1994 Jan;198(1):118–128. doi: 10.1006/viro.1994.1014. [DOI] [PubMed] [Google Scholar]
- Senkevich T. G., Muravnik G. L., Pozdnyakov S. G., Chizhikov V. E., Ryazankina O. I., Shchelkunov S. N., Koonin E. V., Chernos V. I. Nucleotide sequence of XhoI O fragment of ectromelia virus DNA reveals significant differences from vaccinia virus. Virus Res. 1993 Oct;30(1):73–88. doi: 10.1016/0168-1702(93)90017-h. [DOI] [PubMed] [Google Scholar]
- Shchelkunov S. N., Blinov V. M., Sandakhchiev L. S. Genes of variola and vaccinia viruses necessary to overcome the host protective mechanisms. FEBS Lett. 1993 Mar 15;319(1-2):80–83. doi: 10.1016/0014-5793(93)80041-r. [DOI] [PubMed] [Google Scholar]
- Smith G. L. Vaccinia virus glycoproteins and immune evasion. The sixteenth Fleming Lecture. J Gen Virol. 1993 Sep;74(Pt 9):1725–1740. doi: 10.1099/0022-1317-74-9-1725. [DOI] [PubMed] [Google Scholar]
- Tomley F., Binns M., Campbell J., Boursnell M. Sequence analysis of an 11.2 kilobase, near-terminal, BamHI fragment of fowlpox virus. J Gen Virol. 1988 May;69(Pt 5):1025–1040. doi: 10.1099/0022-1317-69-5-1025. [DOI] [PubMed] [Google Scholar]
- Traktman P. Poxviruses: an emerging portrait of biological strategy. Cell. 1990 Aug 24;62(4):621–626. doi: 10.1016/0092-8674(90)90106-o. [DOI] [PubMed] [Google Scholar]
- Turner P. C., Moyer R. W. The molecular pathogenesis of poxviruses. Curr Top Microbiol Immunol. 1990;163:125–151. doi: 10.1007/978-3-642-75605-4_5. [DOI] [PubMed] [Google Scholar]
- Wills A., Delange A. M., Gregson C., Macaulay C., McFadden G. Physical characterization and molecular cloning of the Shope fibroma virus DNA genome. Virology. 1983 Oct 30;130(2):403–414. doi: 10.1016/0042-6822(83)90095-8. [DOI] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]