Abstract
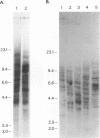
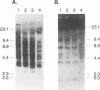
Rapidly progressive T-cell lymphomas were observed in 3 of 10 rhesus monkeys several months after autologous transplantation of enriched bone marrow stem cells that had been transduced with a retroviral vector preparation containing replication-competent virus (R. E. Donahue, S. W. Kessler, D. Bodice, K. McDonagh, C. Dunbar, S. Goodman, B. Agricola, E. Byrne, M. Raffeld, R. Moen, J. Bacher, K. M. Zsebo, and A. W. Nienhuis, J. Exp. Med. 176:1124-1135, 1992). The animals with lymphoma appeared to be tolerant to retroviral antigens in that their sera lacked antibodies reactive with viral proteins and contained 10(4) to 10(5) infectious virus particles per ml. By molecular cloning and DNA sequencing, we have now demonstrated that the serum from one of the monkeys contained a replication-competent retrovirus that arose by recombination between vector and packaging encoding sequences (vector/helper [V/H] recombinant) in the producer clone used for transduction of bone marrow stem cells. Southern blot analysis demonstrated 14 or 25 copies of this genome per cell where present in two animals. The genome of a second replication-competent virus was also recovered by molecular cloning; it arose by recombination involving the genome of the V/H recombinant and endogenous murine retroviral genomes in the producer clone. Twelve copies of this amphotropic virus/mink cell focus-forming virus genome were present in tumor DNA of one animal, but it was not found in tumor DNA of the other two animals with lymphoma. Southern blot analysis of DNA from various tissues demonstrated common insertion site bands in several samples of tumor DNA from one animal, suggesting clonal origin of the lymphoma. Our data are most consistent with a pathogenic mechanism in which chronic productive retroviral infection allowed insertional mutagenesis of critical growth control genes, leading to cell transformation and clonal tumor evolution.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Sakai K., Kitamura N., Nakanishi S., Niwa O., Matsuyama M., Ishimoto A. Characterization of the env gene and long terminal repeat of molecularly cloned Friend mink cell focus-inducing virus DNA. J Virol. 1984 Jun;50(3):813–821. doi: 10.1128/jvi.50.3.813-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick R. K., Kozak S. L., Kabat D. Overcoming interference to retroviral superinfection results in amplified expression and transmission of cloned genes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5404–5408. doi: 10.1073/pnas.85.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., McDonagh K. T., Brandt S. J., Ney P. A., Agricola B., Byrne E., Nienhuis A. W. Development of a high-titer retrovirus producer cell line capable of gene transfer into rhesus monkey hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3738–3742. doi: 10.1073/pnas.87.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. K., Rill D. R., Holladay M. S., Heslop H. E., Moen R. C., Buschle M., Krance R. A., Santana V. M., Anderson W. F., Ihle J. N. Gene marking to determine whether autologous marrow infusion restores long-term haemopoiesis in cancer patients. Lancet. 1993 Nov 6;342(8880):1134–1137. doi: 10.1016/0140-6736(93)92122-a. [DOI] [PubMed] [Google Scholar]
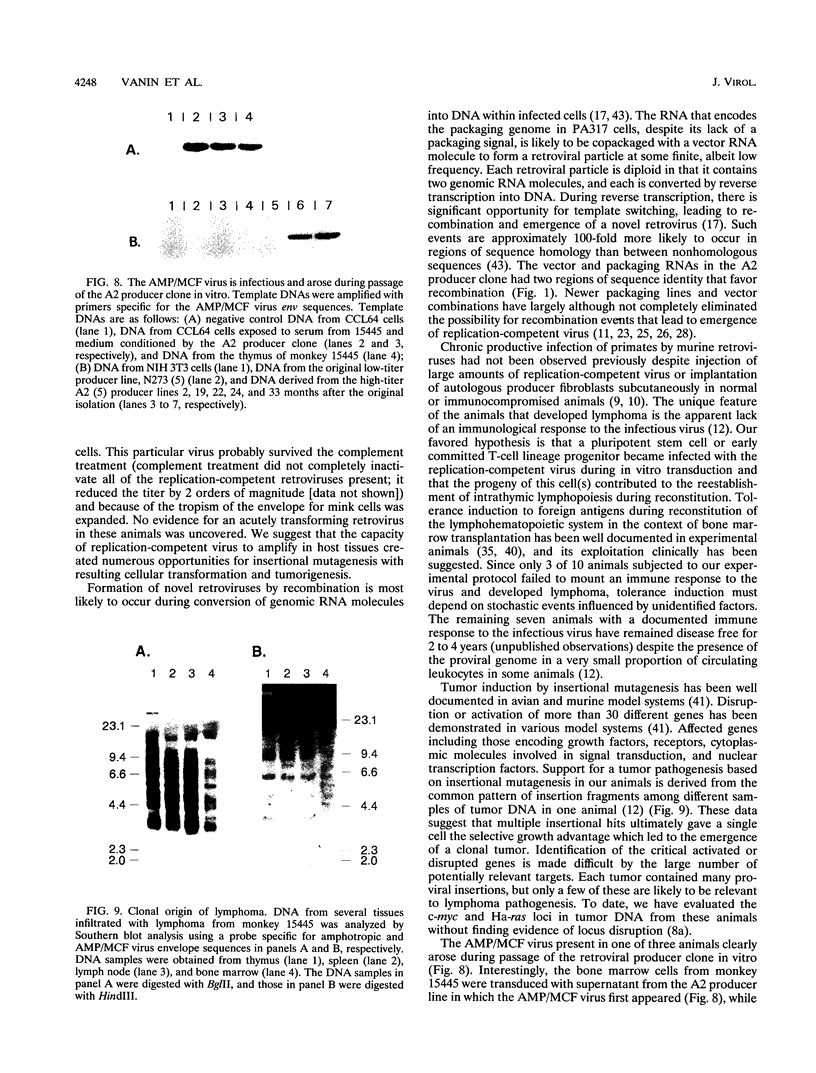
- Brenner M. K., Rill D. R., Moen R. C., Krance R. A., Mirro J., Jr, Anderson W. F., Ihle J. N. Gene-marking to trace origin of relapse after autologous bone-marrow transplantation. Lancet. 1993 Jan 9;341(8837):85–86. doi: 10.1016/0140-6736(93)92560-g. [DOI] [PubMed] [Google Scholar]
- Cornetta K., Moen R. C., Culver K., Morgan R. A., McLachlin J. R., Sturm S., Selegue J., London W., Blaese R. M., Anderson W. F. Amphotropic murine leukemia retrovirus is not an acute pathogen for primates. Hum Gene Ther. 1990 Spring;1(1):15–30. doi: 10.1089/hum.1990.1.1-15. [DOI] [PubMed] [Google Scholar]
- Cornetta K., Morgan R. A., Gillio A., Sturm S., Baltrucki L., O'Reilly R., Anderson W. F. No retroviremia or pathology in long-term follow-up of monkeys exposed to a murine amphotropic retrovirus. Hum Gene Ther. 1991 Fall;2(3):215–219. doi: 10.1089/hum.1991.2.3-215. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue R. E., Kessler S. W., Bodine D., McDonagh K., Dunbar C., Goodman S., Agricola B., Byrne E., Raffeld M., Moen R. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J Exp Med. 1992 Oct 1;176(4):1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzerodt M., Mikkelsen T., Pedersen F. S., Kjeldgaard N. O., Jørgensen P. The nucleotide sequence of the Akv murine leukemia virus genome. Virology. 1984 Apr 15;134(1):196–207. doi: 10.1016/0042-6822(84)90285-x. [DOI] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holland C. A., Wozney J., Hopkins N. Nucleotide sequence of the gp70 gene of murine retrovirus MCF 247. J Virol. 1983 Sep;47(3):413–420. doi: 10.1128/jvi.47.3.413-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Retroviral recombination and reverse transcription. Science. 1990 Nov 30;250(4985):1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- Kelly M., Holland C. A., Lung M. L., Chattopadhyay S. K., Lowy D. R., Hopkins N. H. Nucleotide sequence of the 3' end of MCF 247 murine leukemia virus. J Virol. 1983 Jan;45(1):291–298. doi: 10.1128/jvi.45.1.291-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W., Zimmermann W., Oliff A., Friedrich R. Molecular analysis of the envelope gene and long terminal repeat of Friend mink cell focus-inducing virus: implications for the functions of these sequences. J Virol. 1984 Mar;49(3):828–840. doi: 10.1128/jvi.49.3.828-840.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark G. E., Rapp U. R. Envelope gene sequence of two in vitro-generated mink cell focus-forming murine leukemia viruses which contain the entire gp70 sequence of the endogenous nonecotropic parent. J Virol. 1984 Feb;49(2):530–539. doi: 10.1128/jvi.49.2.530-539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey A. C., Coppola M. A., Thomas C. Y. Origin of pathogenic determinants of recombinant murine leukemia viruses: analysis of Bxv-1-related xenotropic viruses from CWD mice. J Virol. 1990 Nov;64(11):5491–5499. doi: 10.1128/jvi.64.11.5491-5499.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlin J. R., Cornetta K., Eglitis M. A., Anderson W. F. Retroviral-mediated gene transfer. Prog Nucleic Acid Res Mol Biol. 1990;38:91–135. doi: 10.1016/s0079-6603(08)60709-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Retroviral vectors. Curr Top Microbiol Immunol. 1992;158:1–24. doi: 10.1007/978-3-642-75608-5_1. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Noda M., Wagatsuma M., Tamura T., Takano T., Matsubara K. Structure of the baboon endogenous virus genome: cloning of circular virus DNA in bacteriophage lambda. Nucleic Acids Res. 1981 May 11;9(9):2173–2185. doi: 10.1093/nar/9.9.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. R., Buckler C. E., Theodore T. S., Martin M. A., Repaske R. Envelope and long terminal repeat sequences of a cloned infectious NZB xenotropic murine leukemia virus. J Virol. 1985 Jan;53(1):100–106. doi: 10.1128/jvi.53.1.100-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill R. R., Khan A. S., Hoggan M. D., Hartley J. W., Martin M. A., Repaske R. Specific hybridization probes demonstrate fewer xenotropic than mink cell focus-forming murine leukemia virus env-related sequences in DNAs from inbred laboratory mice. J Virol. 1986 May;58(2):359–366. doi: 10.1128/jvi.58.2.359-366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott D., Friedrich R., Rein A. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J Virol. 1990 Feb;64(2):757–766. doi: 10.1128/jvi.64.2.757-766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Pal B. K., Gardner M. B. Characterization of a highly oncogenic murine leukemia virus from wild mice. Int J Cancer. 1982 Mar 15;29(3):345–350. doi: 10.1002/ijc.2910290319. [DOI] [PubMed] [Google Scholar]
- Sachs D. H. Specific transplantation tolerance. N Engl J Med. 1991 Oct 24;325(17):1240–1242. doi: 10.1056/NEJM199110243251708. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Speck N. A., Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987 Mar;7(3):1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck N. A., Renjifo B., Hopkins N. Point mutations in the Moloney murine leukemia virus enhancer identify a lymphoid-specific viral core motif and 1,3-phorbol myristate acetate-inducible element. J Virol. 1990 Feb;64(2):543–550. doi: 10.1128/jvi.64.2.543-550.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. Polymorphism of murine endogenous proviruses revealed by using virus class-specific oligonucleotide probes. J Virol. 1988 Jan;62(1):168–175. doi: 10.1128/jvi.62.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes M., Sachs D. H., Nienhuis A. W., Pearson D. A., Moulton A. D., Bodine D. M. Specific prolongation of skin graft survival following retroviral transduction of bone marrow with an allogeneic major histocompatibility complex gene. Transplantation. 1993 Jan;55(1):197–202. doi: 10.1097/00007890-199301000-00037. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Lazo P. A. Virus-host interactions and the pathogenesis of murine and human oncogenic retroviruses. Curr Top Microbiol Immunol. 1991;171:95–171. doi: 10.1007/978-3-642-76524-7_5. [DOI] [PubMed] [Google Scholar]
- Vogt M., Haggblom C., Swift S., Haas M. Envelope gene and long terminal repeat determine the different biological properties of Rauscher, Friend, and Moloney mink cell focus-inducing viruses. J Virol. 1985 Jul;55(1):184–192. doi: 10.1128/jvi.55.1.184-192.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Temin H. M. Rate and mechanism of nonhomologous recombination during a single cycle of retroviral replication. Science. 1993 Jan 8;259(5092):234–238. doi: 10.1126/science.8421784. [DOI] [PubMed] [Google Scholar]