Abstract
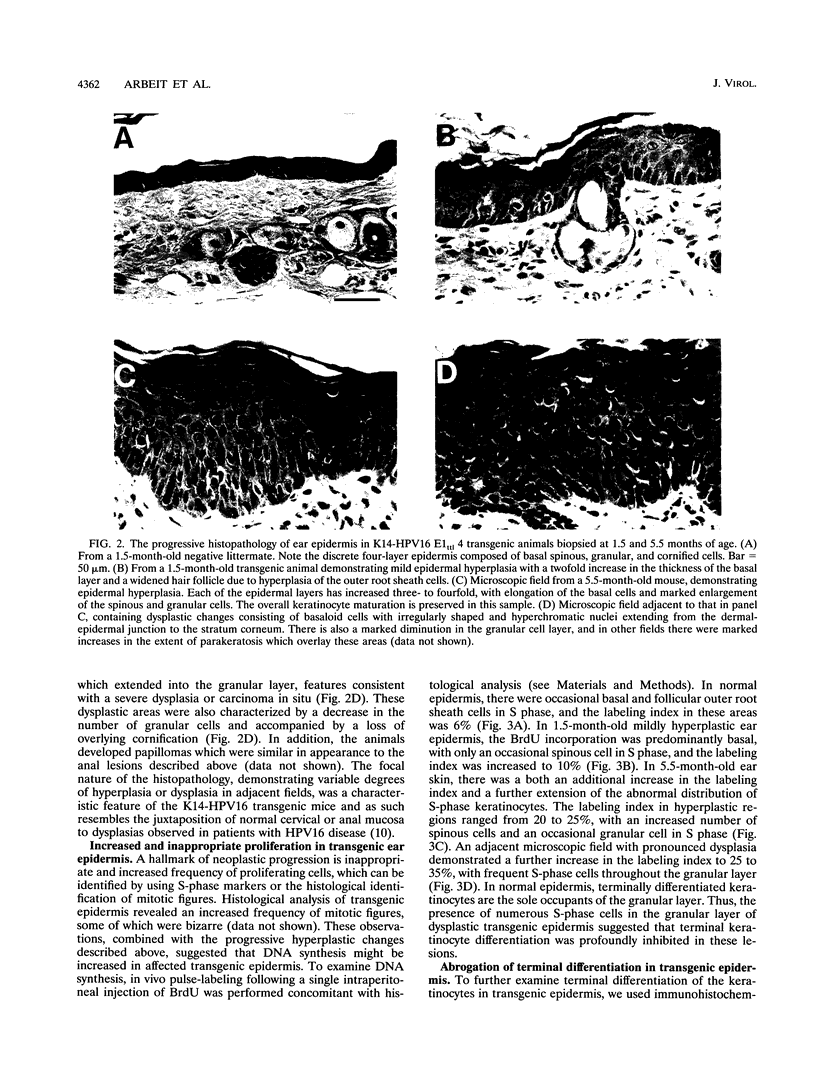
To model human papillomavirus-induced neoplastic progression, expression of the early region of human papillomavirus type 16 (HPV16) was targeted to the basal cells of the squamous epithelium in transgenic mice, using a human keratin 14 (K14) enhancer/promoter. Twenty-one transgenic founder mice were produced, and eight lines carrying either wild-type or mutant HPV16 early regions that did not express the E1 or E2 genes were established. As is characteristic of human cancers, the E6 and E7 genes remained intact in these mutants. The absence of E1 or E2 function did not influence the severity of the phenotype that eventually developed in the transgenic mice. Hyperplasia, papillomatosis, and dysplasia appeared at multiple epidermal and squamous mucosal sites, including ear and truncal skin, face, snout and eyelids, and anus. The ears were the most consistently affected site, with pathology being present in all lines with 100% penetrance. This phenotype also progressed through discernible stages. An initial mild hyperplasia was followed by hyperplasia, which further progressed to dysplasia and papillomatosis. During histopathological progression, there was an incremental increase in cellular DNA synthesis, determined by 5-bromo-2'-deoxyuridine incorporation, and a profound perturbation in keratinocyte terminal differentiation, as revealed by immunohistochemistry to K5, K14, and K10 and filaggrin. These K14-HPV16 transgenic mice present an opportunity to study the role of the HPV16 oncogenes in the neoplastic progression of squamous epithelium and provide a model with which to identify genetic and epigenetic factors necessary for carcinogenesis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit J. M., Münger K., Howley P. M., Hanahan D. Neuroepithelial carcinomas in mice transgenic with human papillomavirus type 16 E6/E7 ORFs. Am J Pathol. 1993 Apr;142(4):1187–1197. [PMC free article] [PubMed] [Google Scholar]
- Bailleul B., Surani M. A., White S., Barton S. C., Brown K., Blessing M., Jorcano J., Balmain A. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell. 1990 Aug 24;62(4):697–708. doi: 10.1016/0092-8674(90)90115-u. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Phelps W. C., Lindgren V., Braun M. J., Gonda M. A., Howley P. M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S., Kraus V. B., Kroger B., Munger K., Howley P. M., Phelps W. C., Nevins J. R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Turksen K., Yu Q. C., Schreiber H., Teng M., Fuchs E. Cachexia and graft-vs.-host-disease-type skin changes in keratin promoter-driven TNF alpha transgenic mice. Genes Dev. 1992 Aug;6(8):1444–1456. doi: 10.1101/gad.6.8.1444. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cintorino M., Syrjänen S., Leoncini P., Bellizzi De Marco E., Petracca R., Pallini V., Tosi P., Mäntyjärvi R., Syrjänen K. Altered expression of filaggrin in human papillomavirus (HPV) lesions of the uterine cervix. Arch Gynecol Obstet. 1988;241(4):235–247. doi: 10.1007/BF00931354. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G., Sun T. T., Lavker R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990 Jun 29;61(7):1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Ikenberg H., Richart R. M., Gissman L. Human papillomavirus type 16 and early cervical neoplasia. N Engl J Med. 1984 Apr 5;310(14):880–883. doi: 10.1056/NEJM198404053101403. [DOI] [PubMed] [Google Scholar]
- Cullen A. P., Reid R., Campion M., Lörincz A. T. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991 Feb;65(2):606–612. doi: 10.1128/jvi.65.2.606-612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio A. M., Romanczuk H., Howley P. M., Baker C. C. Transient replication of human papillomavirus DNAs. J Virol. 1992 Oct;66(10):5949–5958. doi: 10.1128/jvi.66.10.5949-5958.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Goeddel D. V., Ullrich A., Gutterman J. U., Williams R. D., Bringman T. S., Berger W. H. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987 Feb 1;47(3):707–712. [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Guida P., Münger K., Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992 Dec;66(12):6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Eckert R. L. Structure, function, and differentiation of the keratinocyte. Physiol Rev. 1989 Oct;69(4):1316–1346. doi: 10.1152/physrev.1989.69.4.1316. [DOI] [PubMed] [Google Scholar]
- Fischer S. M., O'Connell J. F., Conti C. J., Tacker K. C., Fries J. W., Patrick K. E., Adams L. M., Slaga T. J. Characterization of an inbred strain of the SENCAR mouse that is highly sensitive to phorbol esters. Carcinogenesis. 1987 Mar;8(3):421–424. doi: 10.1093/carcin/8.3.421. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. A., Quintanilla M. I., Orengo C. C., Barber J. L., Eckhardt J. N., Rothnagel J. A., Roop D. R. Cooperation between v-fos and v-rasHA induces autonomous papillomas in transgenic epidermis but not malignant conversion. Cancer Res. 1993 Nov 1;53(21):5071–5075. [PubMed] [Google Scholar]
- Greenhalgh D. A., Rothnagel J. A., Quintanilla M. I., Orengo C. C., Gagne T. A., Bundman D. S., Longley M. A., Roop D. R. Induction of epidermal hyperplasia, hyperkeratosis, and papillomas in transgenic mice by a targeted v-Ha-ras oncogene. Mol Carcinog. 1993;7(2):99–110. doi: 10.1002/mc.2940070208. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. A., Rothnagel J. A., Wang X. J., Quintanilla M. I., Orengo C. C., Gagne T. A., Bundman D. S., Longley M. A., Fisher C., Roop D. R. Hyperplasia, hyperkeratosis and benign tumor production in transgenic mice by a targeted v-fos oncogene suggest a role for fos in epidermal differentiation and neoplasia. Oncogene. 1993 Aug;8(8):2145–2157. [PubMed] [Google Scholar]
- Greenhalgh D. A., Welty D. J., Player A., Yuspa S. H. Two oncogenes, v-fos and v-ras, cooperate to convert normal keratinocytes to squamous cell carcinoma. Proc Natl Acad Sci U S A. 1990 Jan;87(2):643–647. doi: 10.1073/pnas.87.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griep A. E., Herber R., Jeon S., Lohse J. K., Dubielzig R. R., Lambert P. F. Tumorigenicity by human papillomavirus type 16 E6 and E7 in transgenic mice correlates with alterations in epithelial cell growth and differentiation. J Virol. 1993 Mar;67(3):1373–1384. doi: 10.1128/jvi.67.3.1373-1384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Yu Q. C., Fuchs E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 1993 Mar;12(3):973–986. doi: 10.1002/j.1460-2075.1993.tb05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R., Breitburd F., Marche P. N., Orth G. Linkage of regression and malignant conversion of rabbit viral papillomas to MHC class II genes. Nature. 1992 Mar 5;356(6364):66–68. doi: 10.1038/356066a0. [DOI] [PubMed] [Google Scholar]
- Kondoh G., Murata Y., Aozasa K., Yutsudo M., Hakura A. Very high incidence of germ cell tumorigenesis (seminomagenesis) in human papillomavirus type 16 transgenic mice. J Virol. 1991 Jun;65(6):3335–3339. doi: 10.1128/jvi.65.6.3335-3339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R., Fuchs E. A new look into an old problem: keratins as tools to investigate determination, morphogenesis, and differentiation in skin. Genes Dev. 1989 Jan;3(1):1–15. doi: 10.1101/gad.3.1.1. [DOI] [PubMed] [Google Scholar]
- Koutsky L. A., Holmes K. K., Critchlow C. W., Stevens C. E., Paavonen J., Beckmann A. M., DeRouen T. A., Galloway D. A., Vernon D., Kiviat N. B. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992 Oct 29;327(18):1272–1278. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Pan H., Pitot H. C., Liem A., Jackson M., Griep A. E. Epidermal cancer associated with expression of human papillomavirus type 16 E6 and E7 oncogenes in the skin of transgenic mice. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5583–5587. doi: 10.1073/pnas.90.12.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder A., Kuo A., Cardiff R. D., Sinn E., Leder P. v-Ha-ras transgene abrogates the initiation step in mouse skin tumorigenesis: effects of phorbol esters and retinoic acid. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9178–9182. doi: 10.1073/pnas.87.23.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick D. T., Blanton R. A., Gown A. M., McDougall J. K. Altered expression of proliferation and differentiation markers in human papillomavirus 16 and 18 immortalized epithelial cells grown in organotypic culture. Am J Pathol. 1992 Jan;140(1):167–177. [PMC free article] [PubMed] [Google Scholar]
- Münger K., Scheffner M., Huibregtse J. M., Howley P. M. Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products. Cancer Surv. 1992;12:197–217. [PubMed] [Google Scholar]
- Rader J. S., Golub T. R., Hudson J. B., Patel D., Bedell M. A., Laimins L. A. In vitro differentiation of epithelial cells from cervical neoplasias resembles in vivo lesions. Oncogene. 1990 Apr;5(4):571–576. [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Shirasawa H., Tomita Y., Kubota K., Kasai T., Sekiya S., Takamizawa H., Simizu B. Transcriptional differences of the human papillomavirus type 16 genome between precancerous lesions and invasive carcinomas. J Virol. 1988 Mar;62(3):1022–1027. doi: 10.1128/jvi.62.3.1022-1027.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga T. J., Fischer S. M. Strain differences and solvent effects in mouse skin carcinogenesis experiments using carcinogens, tumor initiators and promoters. Prog Exp Tumor Res. 1983;26:85–109. doi: 10.1159/000407254. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Prokoph H., Wettstein F. O. Oncogenic and nononcogenic human genital papillomaviruses generate the E7 mRNA by different mechanisms. J Virol. 1989 Mar;63(3):1441–1447. doi: 10.1128/jvi.63.3.1441-1447.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler A., Kopan R., Duvic M., Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988 Aug;107(2):427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K., Kupper T., Degenstein L., Williams I., Fuchs E. Interleukin 6: insights to its function in skin by overexpression in transgenic mice. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5068–5072. doi: 10.1073/pnas.89.11.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R., Coulombe P. A., Degenstein L., Albers K., Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991 Jan 25;64(2):365–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]
- Vassar R., Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991 May;5(5):714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Vassar R., Hutton M. E., Fuchs E. Transgenic overexpression of transforming growth factor alpha bypasses the need for c-Ha-ras mutations in mouse skin tumorigenesis. Mol Cell Biol. 1992 Oct;12(10):4643–4653. doi: 10.1128/mcb.12.10.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R., Rosenberg M., Ross S., Tyner A., Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1563–1567. doi: 10.1073/pnas.86.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Waggoner S., Barnes W., Stoler M. H., DiPaolo J. A. Human cervical and foreskin epithelial cells immortalized by human papillomavirus DNAs exhibit dysplastic differentiation in vivo. Cancer Res. 1990 Jun 15;50(12):3709–3715. [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in human cancer. Cancer. 1987 May 15;59(10):1692–1696. doi: 10.1002/1097-0142(19870515)59:10<1692::aid-cncr2820591003>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]