Abstract
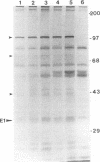
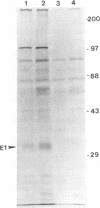
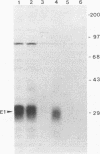
Hepatitis C virus (HCV) accounts for most cases of acute and chronic non-A and non-B hepatitis with serious consequences that may lead to hepatocellular carcinoma. The putative envelope glycoproteins (E1 and E2) of HCV probably play a role in the pathophysiology of the virus. In order to map the immunodominant domains of the E1 glycoprotein, two epitopes from amino acid residues 210 to 223 (P1) and 315 to 327 (P2) were predicted from the HCV sequence. Immunization of mice with the synthetic peptides conjugated to bovine serum albumin induced an antibody response, and the antisera immunoprecipitated the E1 glycoprotein (approximately 33 kDa) of HCV expressed by recombinant vaccinia virus. A panel of HCV-infected human sera was also tested with the synthetic peptides by enzyme-linked immunosorbent assay for epitope-specific responses. Of 38 infected serum samples, 35 (92.1%) demonstrated a spectrum of reactivity to the P2 peptide. On the other hand, only 17 of 38 (44.7%) serum samples were reactive to the P1 peptide. Strains of HCV exhibit a striking genomic diversity. The predicted P1 epitope showed localization in the sequence-variable region, and the P2 epitope localized in a highly conserved domain. Results from this study suggest that the E1 glycoprotein of HCV contains at least two potential antigenic epitopes. Synthetic peptides corresponding to these epitopes and antisera to these peptides may serve as the monospecific immunological reagents to further determine the role of E1 glycoprotein in HCV infection.
Full text
PDF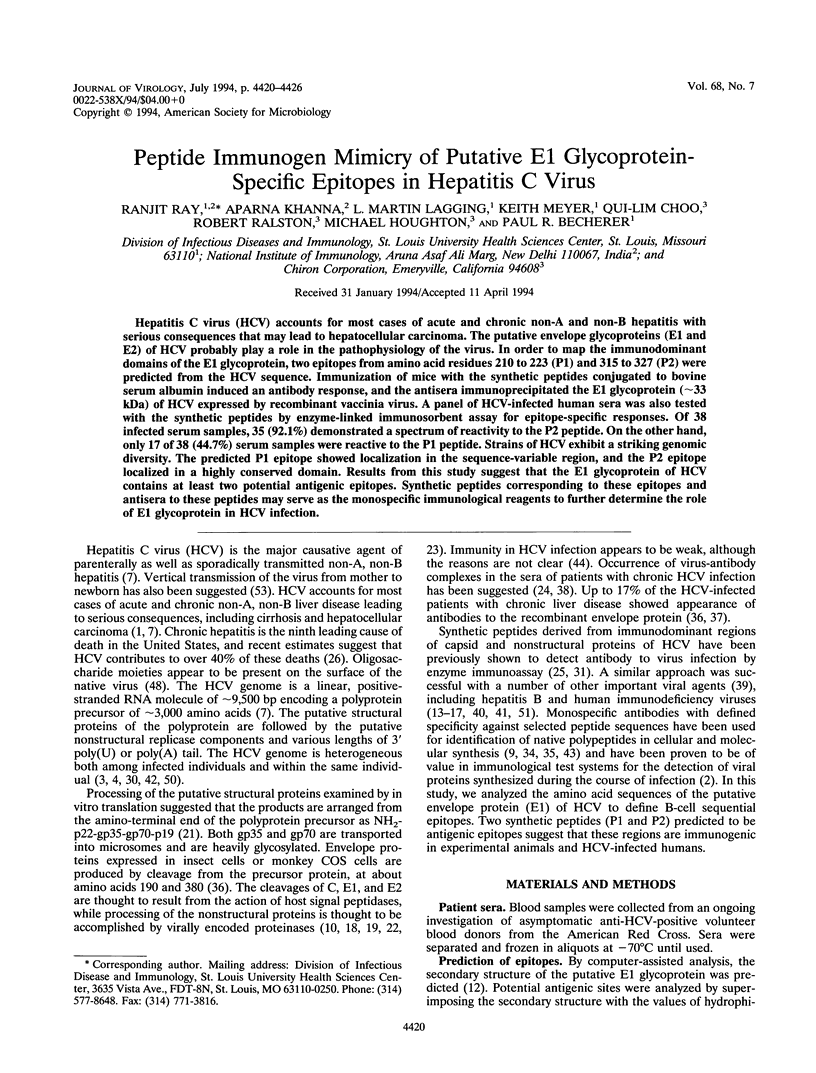

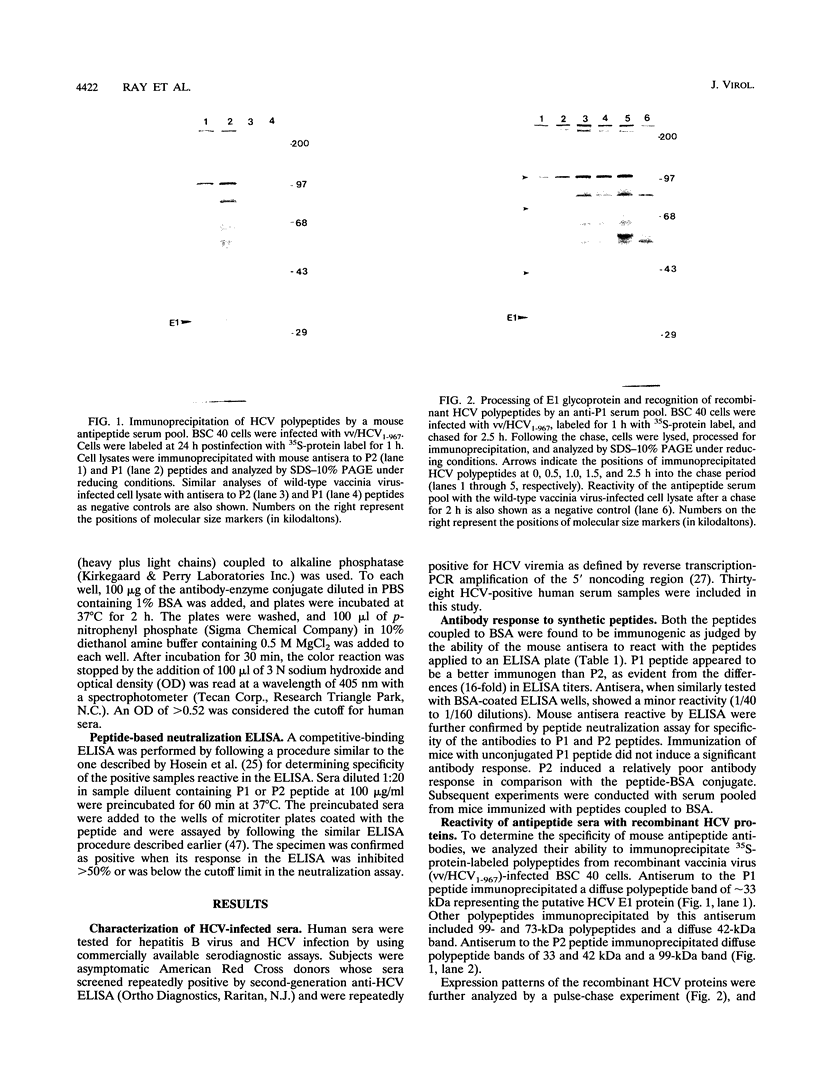
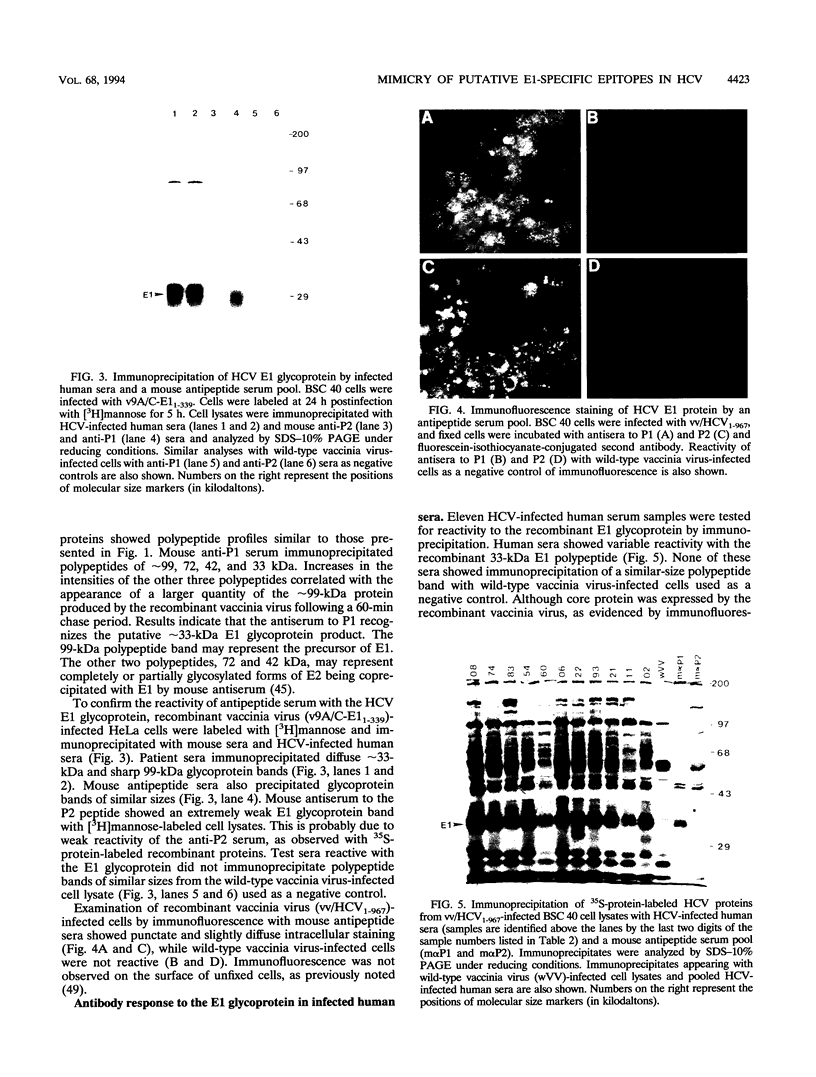
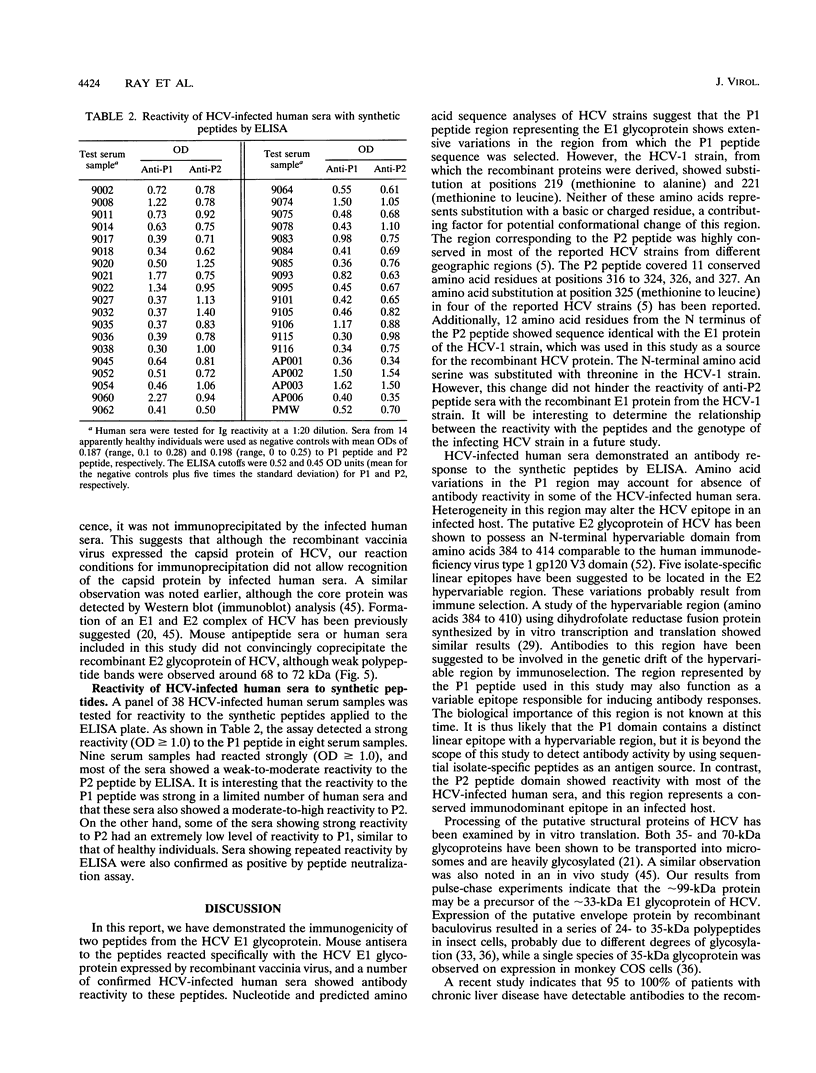


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J., Purcell R. H., Shih J. W., Melpolder J. C., Houghton M., Choo Q. L., Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989 Nov 30;321(22):1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- Brahm J., Vento S., Rondanelli E. G., Ranieri S., Fagan E. A., Williams R., Eddleston A. L. Sequential studies of pre-S2 antigenemia and anti-pre-S2 antibodies in relation to viral replication in acute hepatitis B followed from the early incubation phase. J Med Virol. 1988 Feb;24(2):205–209. doi: 10.1002/jmv.1890240210. [DOI] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. At least 12 genotypes of hepatitis C virus predicted by sequence analysis of the putative E1 gene of isolates collected worldwide. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8234–8238. doi: 10.1073/pnas.90.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. Importance of primer selection for the detection of hepatitis C virus RNA with the polymerase chain reaction assay. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):187–191. doi: 10.1073/pnas.89.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. Sequence analysis of the 5' noncoding region of hepatitis C virus. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4942–4946. doi: 10.1073/pnas.89.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien D. Y., Choo Q. L., Ralston R., Spaete R., Tong M., Houghton M., Kuo G. Persistence of HCV despite antibodies to both putative envelope glycoproteins. Lancet. 1993 Oct 9;342(8876):933–933. doi: 10.1016/0140-6736(93)91983-s. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Eisenberg R. J., Ponce de Leon M., Golub E., Hudecz F., Varrichio A., Cohen G. H. Fine structure analysis of type-specific and type-common antigenic sites of herpes simplex virus glycoprotein D. J Virol. 1984 Nov;52(2):431–435. doi: 10.1128/jvi.52.2.431-435.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckart M. R., Selby M., Masiarz F., Lee C., Berger K., Crawford K., Kuo C., Kuo G., Houghton M., Choo Q. L. The hepatitis C virus encodes a serine protease involved in processing of the putative nonstructural proteins from the viral polyprotein precursor. Biochem Biophys Res Commun. 1993 Apr 30;192(2):399–406. doi: 10.1006/bbrc.1993.1429. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A. 1984 Jan;81(1):140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gerin J. L., Alexander H., Shih J. W., Purcell R. H., Dapolito G., Engle R., Green N., Sutcliffe J. G., Shinnick T. M., Lerner R. A. Chemically synthesized peptides of hepatitis B surface antigen duplicate the d/y specificities and induce subtype-specific antibodies in chimpanzees. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2365–2369. doi: 10.1073/pnas.80.8.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnann J. W., Jr, McCormick J. B., Mitchell S., Nelson J. A., Oldstone M. B. Synthetic peptide immunoassay distinguishes HIV type 1 and HIV type 2 infections. Science. 1987 Sep 11;237(4820):1346–1349. doi: 10.1126/science.2888192. [DOI] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Nelson J. A., Oldstone M. B. Fine mapping of an immunodominant domain in the transmembrane glycoprotein of human immunodeficiency virus. J Virol. 1987 Aug;61(8):2639–2641. doi: 10.1128/jvi.61.8.2639-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Schwimmbeck P. L., Nelson J. A., Truax A. B., Oldstone M. B. Diagnosis of AIDS by using a 12-amino acid peptide representing an immunodominant epitope of the human immunodeficiency virus. J Infect Dis. 1987 Aug;156(2):261–267. doi: 10.1093/infdis/156.2.261. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Boucher C. A., Meloen R. H., Epstein L. G., Smit L., van der Hoek L., Bakker M. Human antibody response to a strain-specific HIV-1 gp120 epitope associated with cell fusion inhibition. AIDS. 1988 Jun;2(3):157–164. [PubMed] [Google Scholar]
- Grakoui A., McCourt D. W., Wychowski C., Feinstone S. M., Rice C. M. A second hepatitis C virus-encoded proteinase. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10583–10587. doi: 10.1073/pnas.90.22.10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., McCourt D. W., Wychowski C., Feinstone S. M., Rice C. M. Characterization of the hepatitis C virus-encoded serine proteinase: determination of proteinase-dependent polyprotein cleavage sites. J Virol. 1993 May;67(5):2832–2843. doi: 10.1128/jvi.67.5.2832-2843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., Wychowski C., Lin C., Feinstone S. M., Rice C. M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993 Mar;67(3):1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Kato N., Ootsuyama Y., Nakagawa M., Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5547–5551. doi: 10.1073/pnas.88.13.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Mizushima H., Akagi T., Mori S., Kakiuchi N., Kato N., Tanaka T., Kimura K., Shimotohno K. Two distinct proteinase activities required for the processing of a putative nonstructural precursor protein of hepatitis C virus. J Virol. 1993 Aug;67(8):4665–4675. doi: 10.1128/jvi.67.8.4665-4675.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Mizushima H., Tanji Y., Komoda Y., Hirowatari Y., Akagi T., Kato N., Kimura K., Shimotohno K. Proteolytic processing and membrane association of putative nonstructural proteins of hepatitis C virus. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10773–10777. doi: 10.1073/pnas.90.22.10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Shimizu Y. K., Kato H., Iwamoto A., Shih J. W., Alter H. J., Purcell R. H., Yoshikura H. Equilibrium centrifugation studies of hepatitis C virus: evidence for circulating immune complexes. J Virol. 1993 Apr;67(4):1953–1958. doi: 10.1128/jvi.67.4.1953-1958.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosein B., Fang C. T., Popovsky M. A., Ye J., Zhang M., Wang C. Y. Improved serodiagnosis of hepatitis C virus infection with synthetic peptide antigen from capsid protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3647–3651. doi: 10.1073/pnas.88.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe G., Abe K., Zebedee S., Nasoff M., Prince A. M. Use of conserved sequences from hepatitis C virus for the detection of viral RNA in infected sera by polymerase chain reaction. Hepatology. 1991 Oct;14(4 Pt 1):595–600. doi: 10.1016/0270-9139(91)90044-v. [DOI] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Kato N., Sekiya H., Ootsuyama Y., Nakazawa T., Hijikata M., Ohkoshi S., Shimotohno K. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993 Jul;67(7):3923–3930. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A., Naik S. R., Thyagarajan S. P., Talwar G. P., Ray R. Seroreactivity and genomic amplification profile of hepatitis C virus from patients with chronic liver disease in India. Am J Med Sci. 1994 Feb;307(2):144–150. doi: 10.1097/00000441-199402000-00014. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Rustgi V. K., Baroudy B. M. Detection of hepatitis C virus-specific antigens in semen from non-A, non-B hepatitis patients. Dig Dis Sci. 1992 May;37(5):641–644. doi: 10.1007/BF01296416. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Notvall L., Chavez D., White R., Frenzel G., Simonsen C., Kim J. Analysis of hepatitis C virus capsid, E1, and E2/NS1 proteins expressed in insect cells. Virology. 1993 Nov;197(1):225–235. doi: 10.1006/viro.1993.1583. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A. Tapping the immunological repertoire to produce antibodies of predetermined specificity. Nature. 1982 Oct 14;299(5884):593–596. doi: 10.1038/299592a0. [DOI] [PubMed] [Google Scholar]
- Manivel V., Panda S. K., Rao K. V. Identification of a new group-specific determinant on hepatitis B surface antigen with a synthetic peptide. J Immunol. 1992 Sep 15;149(6):2082–2088. [PubMed] [Google Scholar]
- Matsuura Y., Harada S., Suzuki R., Watanabe Y., Inoue Y., Saito I., Miyamura T. Expression of processed envelope protein of hepatitis C virus in mammalian and insect cells. J Virol. 1992 Mar;66(3):1425–1431. doi: 10.1128/jvi.66.3.1425-1431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita E., Hayashi N., Ueda K., Kasahara A., Fusamoto H., Takamizawa A., Matsubara K., Okayama H., Kamada T. Expression of MBP-HCV NS1/E2 fusion protein in E. coli and detection of anti-NS1/E2 antibody in type C chronic liver disease. Biochem Biophys Res Commun. 1992 Mar 31;183(3):925–930. doi: 10.1016/s0006-291x(05)80278-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Okamoto H., Sato K., Tanaka T., Mishiro S. Extraordinarily low density of hepatitis C virus estimated by sucrose density gradient centrifugation and the polymerase chain reaction. J Gen Virol. 1992 Mar;73(Pt 3):715–718. doi: 10.1099/0022-1317-73-3-715. [DOI] [PubMed] [Google Scholar]
- Norrby E., Biberfeld G., Chiodi F., von Gegerfeldt A., Nauclér A., Parks E., Lerner R. Discrimination between antibodies to HIV and to related retroviruses using site-directed serology. Nature. 1987 Sep 17;329(6136):248–250. doi: 10.1038/329248a0. [DOI] [PubMed] [Google Scholar]
- Ogata N., Alter H. J., Miller R. H., Purcell R. H. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff E., Mussgay M., Böhm H. O., Schulz G. E., Schaller H. Antibodies against a preselected peptide recognize and neutralize foot and mouth disease virus. EMBO J. 1982;1(7):869–874. doi: 10.1002/j.1460-2075.1982.tb01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A. M., Brotman B., Huima T., Pascual D., Jaffery M., Inchauspé G. Immunity in hepatitis C infection. J Infect Dis. 1992 Mar;165(3):438–443. doi: 10.1093/infdis/165.3.438. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Ikram H., Hopp T. P. Hepatitis B virus vaccine: identification of HBsAg/a and HBsAg/d but not HBsAg/y subtype antigenic determinants on a synthetic immunogenic peptide. Proc Natl Acad Sci U S A. 1982 Jan;79(2):579–582. doi: 10.1073/pnas.79.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston R., Thudium K., Berger K., Kuo C., Gervase B., Hall J., Selby M., Kuo G., Houghton M., Choo Q. L. Characterization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia viruses. J Virol. 1993 Nov;67(11):6753–6761. doi: 10.1128/jvi.67.11.6753-6761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Glaze B. J., Compans R. W. Role of individual glycoproteins of human parainfluenza virus type 3 in the induction of a protective immune response. J Virol. 1988 Mar;62(3):783–787. doi: 10.1128/jvi.62.3.783-787.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Jameel S., Manivel V., Ray R. Indian hepatitis E virus shows a major deletion in the small open reading frame. Virology. 1992 Jul;189(1):359–362. doi: 10.1016/0042-6822(92)90716-3. [DOI] [PubMed] [Google Scholar]
- Sato K., Okamoto H., Aihara S., Hoshi Y., Tanaka T., Mishiro S. Demonstration of sugar moiety on the surface of hepatitis C virions recovered from the circulation of infected humans. Virology. 1993 Sep;196(1):354–357. doi: 10.1006/viro.1993.1488. [DOI] [PubMed] [Google Scholar]
- Selby M. J., Choo Q. L., Berger K., Kuo G., Glazer E., Eckart M., Lee C., Chien D., Kuo C., Houghton M. Expression, identification and subcellular localization of the proteins encoded by the hepatitis C viral genome. J Gen Virol. 1993 Jun;74(Pt 6):1103–1113. doi: 10.1099/0022-1317-74-6-1103. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Kato N., Nakagawa M., Ootsuyama Y., Cho M. J., Nakazawa T., Hijikata M., Ishimura Y., Shimotohno K. Molecular cloning of hepatitis C virus genome from a single Japanese carrier: sequence variation within the same individual and among infected individuals. Virus Res. 1992 Apr;23(1-2):39–53. doi: 10.1016/0168-1702(92)90066-i. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Geysen H. M., Christopherson C., Hall J. E., Mason T. J., Saracco G., Bonino F., Crawford K., Marion C. D., Crawford K. A. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3468–3472. doi: 10.1073/pnas.89.8.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. J., Thaler M. M., Crawford K., Ching K., Kansopon J., Chien D. Y., Hall J. E., Hu F., Houghton M. A unique, predominant hepatitis C virus variant found in an infant born to a mother with multiple variants. J Virol. 1993 Jul;67(7):4365–4368. doi: 10.1128/jvi.67.7.4365-4368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]