Abstract
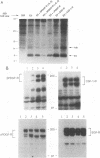
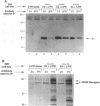
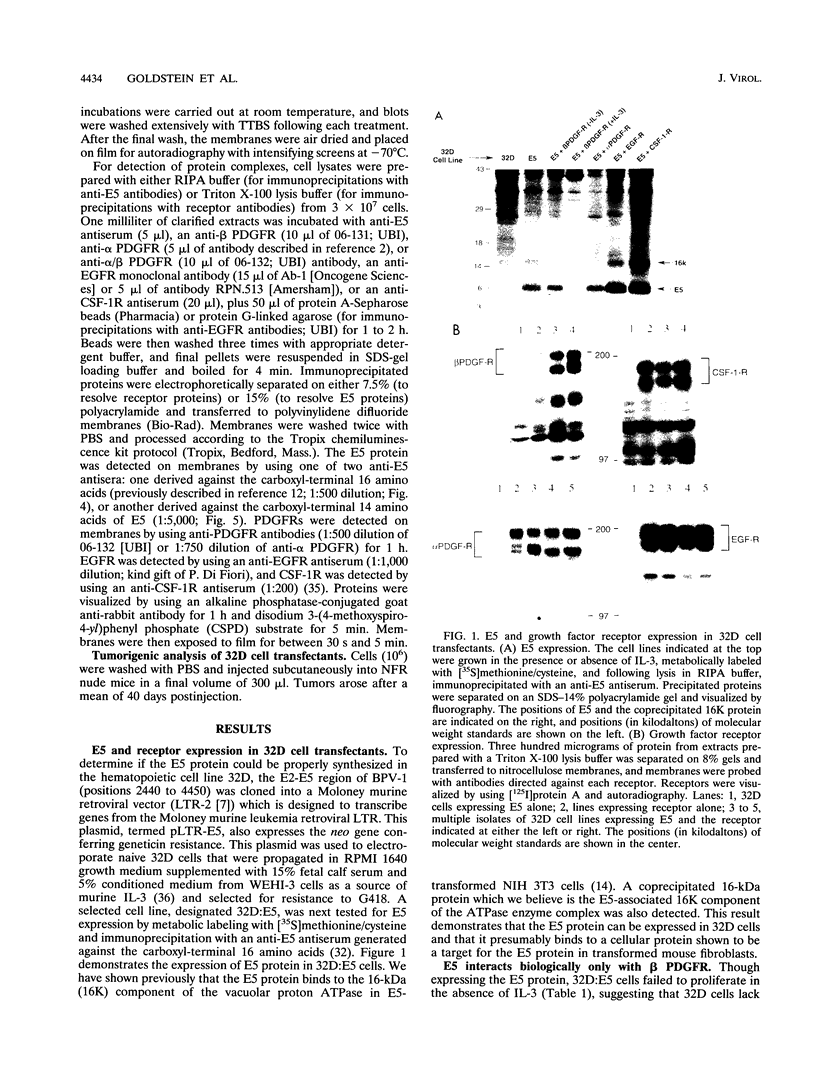
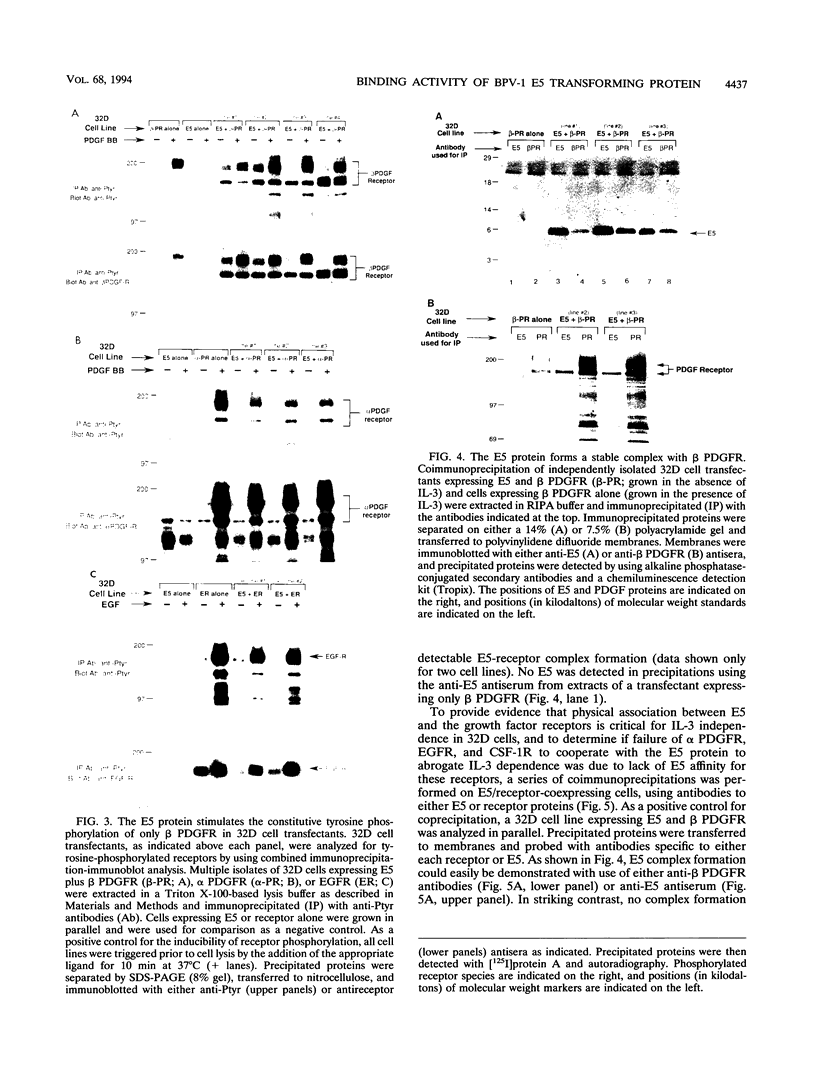
The 44-amino-acid E5 protein of bovine papillomavirus type 1 is a highly hydrophobic protein which appears to transform cells through the activation of growth factor receptors. To investigate the specificity of E5-growth factor receptor interactions required for mitogenic signaling, we utilized a nontumorigenic, murine myeloid cell line (32D) which is strictly dependent on interleukin-3 (IL-3) for sustained proliferation in culture. This IL-3 dependence can be functionally substituted by the expression of a variety of surrogate growth factor receptors and the addition of the corresponding ligand. Several receptor cDNAs for the alpha- and beta-type platelet-derived growth factor receptors [alpha PDGFR and beta PDGFR], the epidermal growth factor receptor, and the colony-stimulating factor 1 receptor) were transfected into 32D cells constitutively expressing the E5 protein to test for IL-3-independent growth. Only beta PDGFR was capable of abrogating the IL-3 dependence of 32D cells. The proliferative signal induced by the coexpression of beta PDGFR and E5 was accompanied by stable complex formation between these proteins, constitutive tyrosine phosphorylation of the receptor, and tumorigenicity in nude mice. The lack of cooperative interaction between E5 and the epidermal growth factor receptor, the colony-stimulating factor 1 receptor, and the highly related alpha PDGFR was paralleled by the inability of E5 to bind to these receptors and failure to increase receptor tyrosine phosphorylation. Thus, these data indicate that the ability of E5 to induce sustained proliferation and transformation of 32D cells is a direct consequence of specific interaction between the E5 protein and the beta PDGFR signaling complex and the subsequent stimulation of receptor tyrosine phosphorylation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Claesson-Welsh L., Eriksson A., Westermark B., Heldin C. H. cDNA cloning and expression of the human A-type platelet-derived growth factor (PDGF) receptor establishes structural similarity to the B-type PDGF receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4917–4921. doi: 10.1073/pnas.86.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L., Hammacher A., Westermark B., Heldin C. H., Nistér M. Identification and structural analysis of the A type receptor for platelet-derived growth factor. Similarities with the B type receptor. J Biol Chem. 1989 Jan 25;264(3):1742–1747. [PubMed] [Google Scholar]
- Cohen B. D., Goldstein D. J., Rutledge L., Vass W. C., Lowy D. R., Schlegel R., Schiller J. T. Transformation-specific interaction of the bovine papillomavirus E5 oncoprotein with the platelet-derived growth factor receptor transmembrane domain and the epidermal growth factor receptor cytoplasmic domain. J Virol. 1993 Sep;67(9):5303–5311. doi: 10.1128/jvi.67.9.5303-5311.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. D., Lowy D. R., Schiller J. T. The conserved C-terminal domain of the bovine papillomavirus E5 oncoprotein can associate with an alpha-adaptin-like molecule: a possible link between growth factor receptors and viral transformation. Mol Cell Biol. 1993 Oct;13(10):6462–6468. doi: 10.1128/mcb.13.10.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P., Lankford S. P., Bonifacino J. S., Klausner R. D. Membrane protein association by potential intramembrane charge pairs. Nature. 1991 May 30;351(6325):414–416. doi: 10.1038/351414a0. [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Fleming T. P., Hazan R., Ullrich A., King C. R., Schlessinger J., Aaronson S. A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- DiMaio D., Guralski D., Schiller J. T. Translation of open reading frame E5 of bovine papillomavirus is required for its transforming activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1797–1801. doi: 10.1073/pnas.83.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Eriksson A., Siegbahn A., Westermark B., Heldin C. H., Claesson-Welsh L. PDGF alpha- and beta-receptors activate unique and common signal transduction pathways. EMBO J. 1992 Feb;11(2):543–550. doi: 10.1002/j.1460-2075.1992.tb05085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Andresson T., Sparkowski J. J., Schlegel R. The BPV-1 E5 protein, the 16 kDa membrane pore-forming protein and the PDGF receptor exist in a complex that is dependent on hydrophobic transmembrane interactions. EMBO J. 1992 Dec;11(13):4851–4859. doi: 10.1002/j.1460-2075.1992.tb05591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Finbow M. E., Andresson T., McLean P., Smith K., Bubb V., Schlegel R. Bovine papillomavirus E5 oncoprotein binds to the 16K component of vacuolar H(+)-ATPases. Nature. 1991 Jul 25;352(6333):347–349. doi: 10.1038/352347a0. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Kulke R., Dimaio D., Schlegel R. A glutamine residue in the membrane-associating domain of the bovine papillomavirus type 1 E5 oncoprotein mediates its binding to a transmembrane component of the vacuolar H(+)-ATPase. J Virol. 1992 Jan;66(1):405–413. doi: 10.1128/jvi.66.1.405-413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Schlegel R. The E5 oncoprotein of bovine papillomavirus binds to a 16 kd cellular protein. EMBO J. 1990 Jan;9(1):137–145. doi: 10.1002/j.1460-2075.1990.tb08089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger J. S., Sakakeeny M. A., Humphries R. K., Eaves C. J., Eckner R. J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci U S A. 1983 May;80(10):2931–2935. doi: 10.1073/pnas.80.10.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff D. E., Lancaster W. D. Genetic analysis of the 3' early region transformation and replication functions of bovine papillomavirus type 1. Virology. 1986 Apr 15;150(1):221–230. doi: 10.1016/0042-6822(86)90281-3. [DOI] [PubMed] [Google Scholar]
- Horwitz B. H., Burkhardt A. L., Schlegel R., DiMaio D. 44-amino-acid E5 transforming protein of bovine papillomavirus requires a hydrophobic core and specific carboxyl-terminal amino acids. Mol Cell Biol. 1988 Oct;8(10):4071–4078. doi: 10.1128/mcb.8.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Sherr C. J. Human colony-stimulating factor 1 (CSF-1) receptor confers CSF-1 responsiveness to interleukin-3-dependent 32DC13 mouse myeloid cells and abrogates differentiation in response to granulocyte CSF. Blood. 1990 May 1;75(9):1780–1787. [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Processing of the platelet-derived growth factor receptor. Biosynthetic and degradation studies using anti-receptor antibodies. J Biol Chem. 1987 Jun 5;262(16):7932–7937. [PubMed] [Google Scholar]
- Klymkowsky M. W., Shook D. R., Maynell L. A. Evidence that the deep keratin filament systems of the Xenopus embryo act to ensure normal gastrulation. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8736–8740. doi: 10.1073/pnas.89.18.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptak C., Ramon y Cajal S., Kulke R., Horwitz B. H., Riese D. J., 2nd, Dotto G. P., DiMaio D. Tumorigenic transformation of murine keratinocytes by the E5 genes of bovine papillomavirus type 1 and human papillomavirus type 16. J Virol. 1991 Dec;65(12):7078–7083. doi: 10.1128/jvi.65.12.7078-7083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Schlessinger J. Platelet-derived growth factor (PDGF)-induced disulfide-linked dimerization of PDGF receptor in living cells. Mol Cell Biol. 1991 Jul;11(7):3756–3761. doi: 10.1128/mcb.11.7.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Heidaran M., Miki T., Popescu N., La Rochelle W., Kraus M., Pierce J., Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989 Feb 10;243(4892):800–804. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- Matsui T., Pierce J. H., Fleming T. P., Greenberger J. S., LaRochelle W. J., Ruggiero M., Aaronson S. A. Independent expression of human alpha or beta platelet-derived growth factor receptor cDNAs in a naive hematopoietic cell leads to functional coupling with mitogenic and chemotactic signaling pathways. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8314–8318. doi: 10.1073/pnas.86.21.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H., Nelson N. Disruption of genes encoding subunits of yeast vacuolar H(+)-ATPase causes conditional lethality. Proc Natl Acad Sci U S A. 1990 May;87(9):3503–3507. doi: 10.1073/pnas.87.9.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. Structure and pharmacology of the proton-ATPases. Trends Pharmacol Sci. 1991 Feb;12(2):71–75. doi: 10.1016/0165-6147(91)90501-i. [DOI] [PubMed] [Google Scholar]
- Nilson L. A., DiMaio D. Platelet-derived growth factor receptor can mediate tumorigenic transformation by the bovine papillomavirus E5 protein. Mol Cell Biol. 1993 Jul;13(7):4137–4145. doi: 10.1128/mcb.13.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H., Siegel J. P., Erdos M., Gnarra J. R., Toledano M. B., Sharon M., Mostowski H., Feinberg M. B., Pierce J. H., Leonard W. J. Interleukin (IL)-2 and IL-3 induce distinct but overlapping responses in murine IL-3-dependent 32D cells transduced with human IL-2 receptor beta chain: involvement of tyrosine kinase(s) other than p56lck. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2789–2793. doi: 10.1073/pnas.89.7.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeler J. S., Donzell W. C., Anderson R. G. The appendage domain of the AP-2 subunit is not required for assembly or invagination of clathrin-coated pits. J Cell Biol. 1993 Jan;120(1):47–54. doi: 10.1083/jcb.120.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., DiMaio D. Specific interaction between the bovine papillomavirus E5 transforming protein and the beta receptor for platelet-derived growth factor in stably transformed and acutely transfected cells. J Virol. 1994 Jun;68(6):3582–3592. doi: 10.1128/jvi.68.6.3582-3592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., Nilson L. A., DiMaio D. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 1991 Apr;10(4):845–855. doi: 10.1002/j.1460-2075.1991.tb08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Di Marco E., Cox G. W., Lombardi D., Ruggiero M., Varesio L., Wang L. M., Choudhury G. G., Sakaguchi A. Y., Di Fiore P. P. Macrophage-colony-stimulating factor (CSF-1) induces proliferation, chemotaxis, and reversible monocytic differentiation in myeloid progenitor cells transfected with the human c-fms/CSF-1 receptor cDNA. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5613–5617. doi: 10.1073/pnas.87.15.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Ruggiero M., Fleming T. P., Di Fiore P. P., Greenberger J. S., Varticovski L., Schlessinger J., Rovera G., Aaronson S. A. Signal transduction through the EGF receptor transfected in IL-3-dependent hematopoietic cells. Science. 1988 Feb 5;239(4840):628–631. doi: 10.1126/science.3257584. [DOI] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Vousden K. H., Lowy D. R. E5 open reading frame of bovine papillomavirus type 1 encodes a transforming gene. J Virol. 1986 Jan;57(1):1–6. doi: 10.1128/jvi.57.1.1-6.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Wade-Glass M., Rabson M. S., Yang Y. C. The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science. 1986 Jul 25;233(4762):464–467. doi: 10.1126/science.3014660. [DOI] [PubMed] [Google Scholar]
- Settleman J., DiMaio D. Efficient transactivation and morphologic transformation by bovine papillomavirus genes expressed from a bovine papillomavirus/simian virus 40 recombinant virus. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9007–9011. doi: 10.1073/pnas.85.23.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]