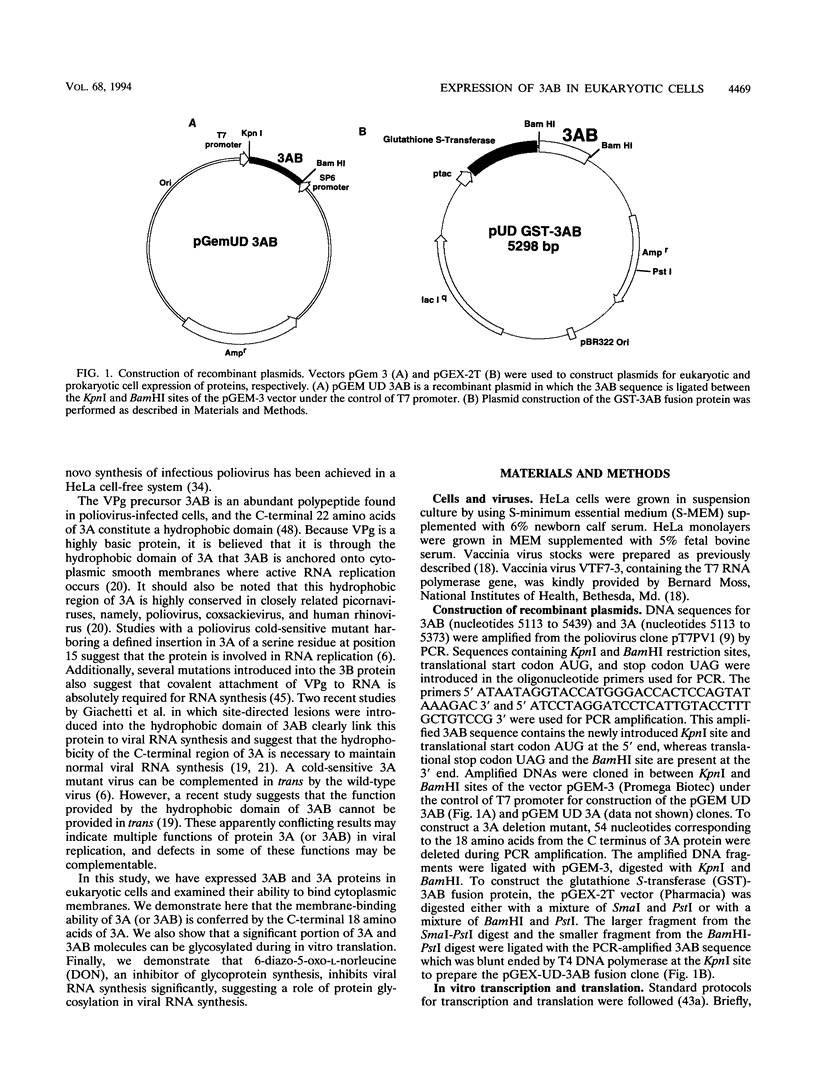
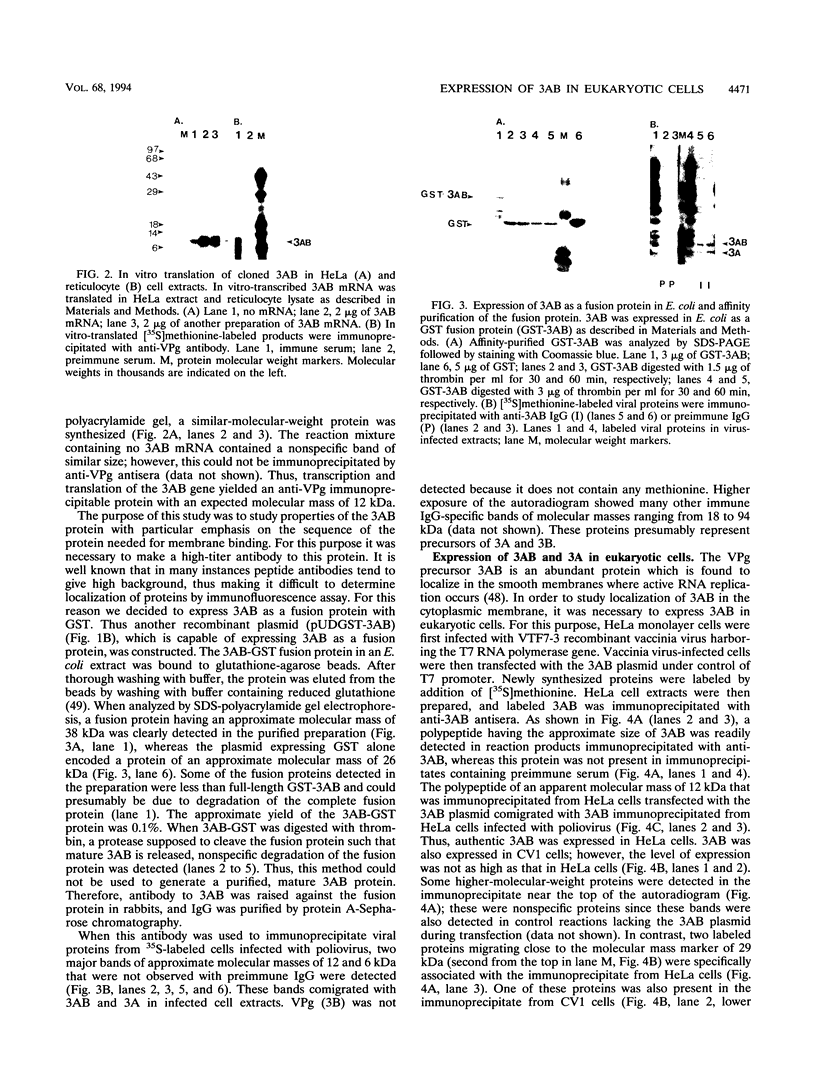
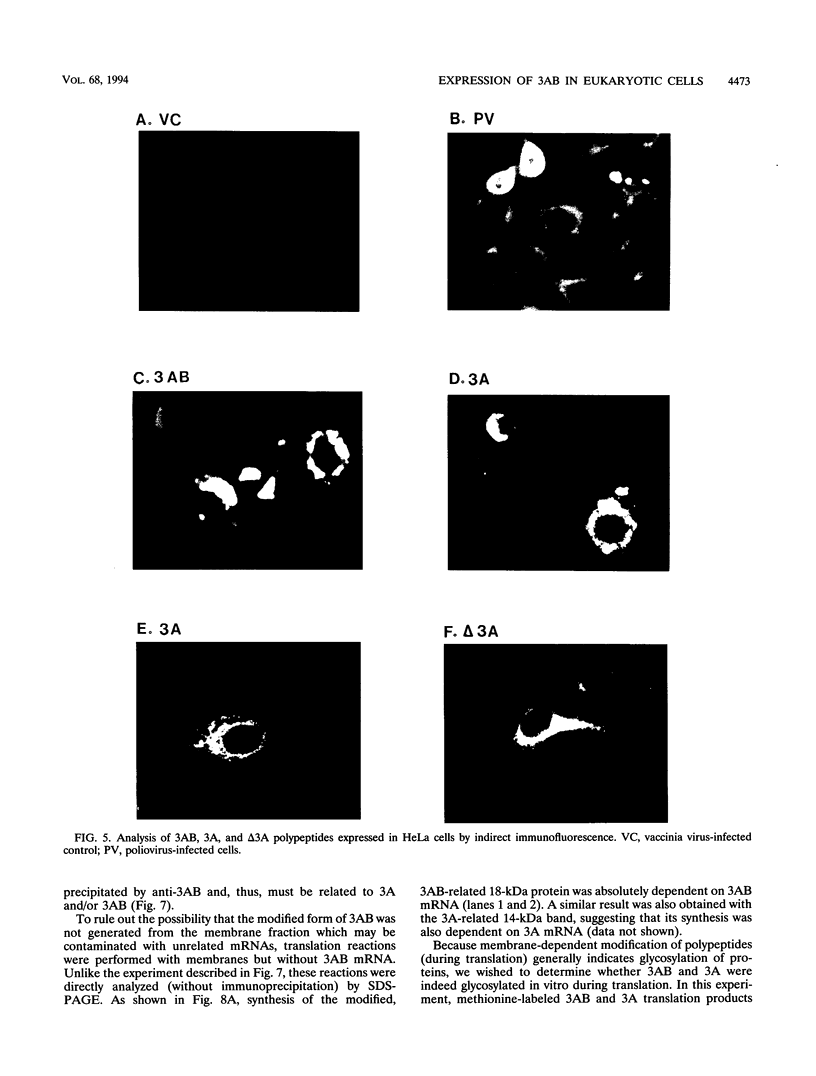
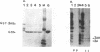Abstract
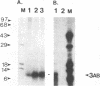
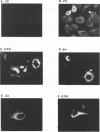
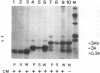
The poliovirus-encoded, membrane-associated VPg-precursor polypeptide 3AB has been implicated in the initiation of viral RNA synthesis. We have expressed 3AB and 3A polypeptides in eukaryotic cells and examined their localization using indirect immunofluorescence and a direct in vitro membrane-binding assay. Results presented here demonstrate that both 3AB and 3A are capable of localizing in the endoplasmic reticulum and the Golgi apparatus in transfected HeLa cells in the absence of any other poliovirus protein. We have also shown that the carboxy-terminal 18 amino acids of 3A that constitute an amphipathic domain are important in membrane binding of 3A and 3AB. Additionally, we demonstrate that a significant fraction of both 3A and 3AB can be glycosylated in a membrane-dependent fashion during in vitro translation in reticulocyte lysate. We demonstrate that 6-diazo-5-oxo-L-norleucine, an inhibitor of glycoprotein synthesis, significantly inhibits poliovirus RNA synthesis in vivo. The implications of glycosylation of 3AB (and 3A) in viral replication are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andino R., Rieckhof G. E., Achacoso P. L., Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5'-end of viral RNA. EMBO J. 1993 Sep;12(9):3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andino R., Rieckhof G. E., Baltimore D. A functional ribonucleoprotein complex forms around the 5' end of poliovirus RNA. Cell. 1990 Oct 19;63(2):369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- Andrews N. C., Baltimore D. Purification of a terminal uridylyltransferase that acts as host factor in the in vitro poliovirus replicase reaction. Proc Natl Acad Sci U S A. 1986 Jan;83(2):221–225. doi: 10.1073/pnas.83.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. Anti-VPg antibody inhibition of the poliovirus replicase reaction and production of covalent complexes of VPg-related proteins and RNA. Cell. 1982 Oct;30(3):745–752. doi: 10.1016/0092-8674(82)90279-3. [DOI] [PubMed] [Google Scholar]
- Barton D. J., Flanegan J. B. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J Virol. 1993 Feb;67(2):822–831. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein H. D., Sarnow P., Baltimore D. Genetic complementation among poliovirus mutants derived from an infectious cDNA clone. J Virol. 1986 Dec;60(3):1040–1049. doi: 10.1128/jvi.60.3.1040-1049.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstein H. D., Baltimore D. Poliovirus mutant that contains a cold-sensitive defect in viral RNA synthesis. J Virol. 1988 Aug;62(8):2922–2928. doi: 10.1128/jvi.62.8.2922-2928.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz K., Egger D., Troxler M., Pasamontes L. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J Virol. 1990 Mar;64(3):1156–1163. doi: 10.1128/jvi.64.3.1156-1163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charini W. A., Burns C. C., Ehrenfeld E., Semler B. L. trans rescue of a mutant poliovirus RNA polymerase function. J Virol. 1991 May;65(5):2655–2665. doi: 10.1128/jvi.65.5.2655-2665.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M. W., Richards O. C., Dmitrieva T. M., Agol V., Ehrenfeld E. RNA duplex unwinding activity of poliovirus RNA-dependent RNA polymerase 3Dpol. J Virol. 1993 Jun;67(6):3010–3018. doi: 10.1128/jvi.67.6.3010-3018.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M., Newman J. F., Filman D., Hogle J. M., Rowlands D. J., Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987 Jun 11;327(6122):482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- Clark M. E., Lieberman P. M., Berk A. J., Dasgupta A. Direct cleavage of human TATA-binding protein by poliovirus protease 3C in vivo and in vitro. Mol Cell Biol. 1993 Feb;13(2):1232–1237. doi: 10.1128/mcb.13.2.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. M., Baltimore D. Genome-linked protein VPg of poliovirus is present as free VPg and VPg-pUpU in poliovirus-infected cells. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7452–7455. doi: 10.1073/pnas.80.24.7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A. Purification of host factor required for in vitro transcription of poliovirus RNA. Virology. 1983 Jul 15;128(1):245–251. doi: 10.1016/0042-6822(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus polyuridylic acid polymerase and RNA replicase have the same viral polypeptide. J Virol. 1979 Jan;29(1):352–360. doi: 10.1128/jvi.29.1.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachetti C., Hwang S. S., Semler B. L. cis-acting lesions targeted to the hydrophobic domain of a poliovirus membrane protein involved in RNA replication. J Virol. 1992 Oct;66(10):6045–6057. doi: 10.1128/jvi.66.10.6045-6057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachetti C., Semler B. L. Role of a viral membrane polypeptide in strand-specific initiation of poliovirus RNA synthesis. J Virol. 1991 May;65(5):2647–2654. doi: 10.1128/jvi.65.5.2647-2654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Guskey L. E. Poliovirus and vesicular stomatitis virus replication in the presence of 6-diazo-5-oxo-L-norleucine or 2-deoxy-D-glucose. J Med Virol. 1984;14(2):159–167. doi: 10.1002/jmv.1890140210. [DOI] [PubMed] [Google Scholar]
- Hey T. D., Richards O. C., Ehrenfeld E. Host factor-induced template modification during synthesis of poliovirus RNA in vitro. J Virol. 1987 Mar;61(3):802–811. doi: 10.1128/jvi.61.3.802-811.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irurzun A., Perez L., Carrasco L. Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A. Virology. 1992 Nov;191(1):166–175. doi: 10.1016/0042-6822(92)90178-r. [DOI] [PubMed] [Google Scholar]
- Johnson K. L., Sarnow P. Three poliovirus 2B mutants exhibit noncomplementable defects in viral RNA amplification and display dosage-dependent dominance over wild-type poliovirus. J Virol. 1991 Aug;65(8):4341–4349. doi: 10.1128/jvi.65.8.4341-4349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lama J., Carrasco L. Expression of poliovirus nonstructural proteins in Escherichia coli cells. Modification of membrane permeability induced by 2B and 3A. J Biol Chem. 1992 Aug 5;267(22):15932–15937. [PubMed] [Google Scholar]
- Li J. P., Baltimore D. Isolation of poliovirus 2C mutants defective in viral RNA synthesis. J Virol. 1988 Nov;62(11):4016–4021. doi: 10.1128/jvi.62.11.4016-4021.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinski J. M., Kaplan G., Racaniello V. R., Dasgupta A. Mechanism of in vitro synthesis of covalently linked dimeric RNA molecules by the poliovirus replicase. J Virol. 1986 May;58(2):459–467. doi: 10.1128/jvi.58.2.459-467.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinski J. M., Ransone L. J., Dasgupta A. Primer-dependent synthesis of covalently linked dimeric RNA molecules by poliovirus replicase. J Virol. 1987 Oct;61(10):2997–3003. doi: 10.1128/jvi.61.10.2997-3003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynell L. A., Kirkegaard K., Klymkowsky M. W. Inhibition of poliovirus RNA synthesis by brefeldin A. J Virol. 1992 Apr;66(4):1985–1994. doi: 10.1128/jvi.66.4.1985-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi Y., Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986 Aug 25;261(24):11398–11403. [PubMed] [Google Scholar]
- Molla A., Paul A. V., Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991 Dec 13;254(5038):1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- Morrow C. D., Dasgupta A. Antibody to a synthetic nonapeptide corresponding to the NH2 terminus of poliovirus genome-linked protein VPg reacts with native VPg and inhibits in vitro replication of poliovirus RNA. J Virol. 1983 Nov;48(2):429–439. doi: 10.1128/jvi.48.2.429-439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C. D., Hocko J., Navab M., Dasgupta A. ATP is required for initiation of poliovirus RNA synthesis in vitro: demonstration of tyrosine-phosphate linkage between in vitro-synthesized RNA and genome-linked protein. J Virol. 1984 May;50(2):515–523. doi: 10.1128/jvi.50.2.515-523.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C. D., Warren B., Lentz M. R. Expression of enzymatically active poliovirus RNA-dependent RNA polymerase in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6050–6054. doi: 10.1073/pnas.84.17.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld K. L., Richards O. C., Ehrenfeld E. Expression and characterization of poliovirus proteins 3BVPg, 3Cpro, and 3Dpol in recombinant baculovirus-infected Spodoptera frugiperda cells. Virus Res. 1991 May;19(2-3):173–188. doi: 10.1016/0168-1702(91)90044-v. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- Oda K., Fujiwara T., Ikehara Y. Brefeldin A arrests the intracellular transport of viral envelope proteins in primary cultured rat hepatocytes and HepG2 cells. Biochem J. 1990 Jan 1;265(1):161–167. doi: 10.1042/bj2650161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus S. E., Diamond D. C., Emini E. A., Wimmer E. Guanidine-selected mutants of poliovirus: mapping of point mutations to polypeptide 2C. J Virol. 1986 Feb;57(2):638–646. doi: 10.1128/jvi.57.2.638-646.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus S. E., Wimmer E. Production of guanidine-resistant and -dependent poliovirus mutants from cloned cDNA: mutations in polypeptide 2C are directly responsible for altered guanidine sensitivity. J Virol. 1986 Nov;60(2):793–796. doi: 10.1128/jvi.60.2.793-796.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Palant O., Gluzman Y. Purification and properties of poliovirus RNA polymerase expressed in Escherichia coli. J Virol. 1989 Jan;63(1):216–225. doi: 10.1128/jvi.63.1.216-225.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuer Q., Kuhn R. J., Wimmer E. Characterization of poliovirus clones containing lethal and nonlethal mutations in the genome-linked protein VPg. J Virol. 1990 Jun;64(6):2967–2975. doi: 10.1128/jvi.64.6.2967-2975.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez P. L., Carrasco L. Poliovirus protein 2C has ATPase and GTPase activities. J Biol Chem. 1993 Apr 15;268(11):8105–8110. [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Hanecak R., Dorner L. F., Wimmer E. A membrane-associated precursor to poliovirus VPg identified by immunoprecipitation with antibodies directed against a synthetic heptapeptide. Cell. 1982 Feb;28(2):405–412. doi: 10.1016/0092-8674(82)90358-0. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Takeda N., Kuhn R. J., Yang C. F., Takegami T., Wimmer E. Initiation of poliovirus plus-strand RNA synthesis in a membrane complex of infected HeLa cells. J Virol. 1986 Oct;60(1):43–53. doi: 10.1128/jvi.60.1.43-53.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegami T., Kuhn R. J., Anderson C. W., Wimmer E. Membrane-dependent uridylylation of the genome-linked protein VPg of poliovirus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7447–7451. doi: 10.1073/pnas.80.24.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegami T., Semler B. L., Anderson C. W., Wimmer E. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology. 1983 Jul 15;128(1):33–47. doi: 10.1016/0042-6822(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Tobin G. J., Young D. C., Flanegan J. B. Self-catalyzed linkage of poliovirus terminal protein VPg to poliovirus RNA. Cell. 1989 Nov 3;59(3):511–519. doi: 10.1016/0092-8674(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. A., Flanegan J. B. Identification of poliovirus polypeptide P63 as a soluble RNA-dependent RNA polymerase. J Virol. 1980 Sep;35(3):732–740. doi: 10.1128/jvi.35.3.732-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Dewalt P. G., Johnson V. H., Lamb J. G., Semler B. L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology. 1988 Sep;166(1):265–270. doi: 10.1016/0042-6822(88)90172-9. [DOI] [PubMed] [Google Scholar]