Abstract
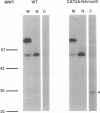
In this report we describe the rescue of a transfectant influenza A virus which stably expresses a heterologous protein, bacterial chloramphenicol acetyltransferase (CAT). The foreign sequences encoding CAT are expressed as part of an essential influenza virus segment, that coding for the neuraminidase (NA) protein. The novel way by which this was achieved involved inserting in frame the 16-amino-acid self-cleaving 2A protease of foot-and-mouth disease virus between the CAT and the NA coding sequences. The resultant gene produces a polyprotein which is proteolytically cleaved to release both CAT and NA. The intramolecular cleavage occurs at the C terminus of the 2A sequence between a glycine-proline dipeptide motif such that the released NA protein has an additional N-terminal proline residue. The transfectant virus is stable upon passage in tissue culture. CAT activity is expressed at high levels in cell culture supernatants and in the allantoic fluid of infected eggs. Since the chimeric segment must maintain the heterologous reading frame to retain viability, the virus stability is dependent upon concomitant synthesis of the heterologous protein. This design may be particularly appropriate for utilization of influenza virus as a mammalian expression vector.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilsel P., Castrucci M. R., Kawaoka Y. Mutations in the cytoplasmic tail of influenza A virus neuraminidase affect incorporation into virions. J Virol. 1993 Nov;67(11):6762–6767. doi: 10.1128/jvi.67.11.6762-6767.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrucci M. R., Bilsel P., Kawaoka Y. Attenuation of influenza A virus by insertion of a foreign epitope into the neuraminidase. J Virol. 1992 Aug;66(8):4647–4653. doi: 10.1128/jvi.66.8.4647-4653.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrucci M. R., Kawaoka Y. Biologic importance of neuraminidase stalk length in influenza A virus. J Virol. 1993 Feb;67(2):759–764. doi: 10.1128/jvi.67.2.759-764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami M., Luytjes W., Krystal M., Palese P. Introduction of site-specific mutations into the genome of influenza virus. Proc Natl Acad Sci U S A. 1990 May;87(10):3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami M., Palese P. High-efficiency formation of influenza virus transfectants. J Virol. 1991 May;65(5):2711–2713. doi: 10.1128/jvi.65.5.2711-2713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Q., Schulman J. L., Moran T., Bona C., Palese P. Influenza A virus transfectants with chimeric hemagglutinins containing epitopes from different subtypes. J Virol. 1992 Jan;66(1):399–404. doi: 10.1128/jvi.66.1.399-404.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Palese P. Characterization of the polyadenylation signal of influenza virus RNA. J Virol. 1994 Feb;68(2):1245–1249. doi: 10.1128/jvi.68.2.1245-1249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Palese P. Mutational analysis of the promoter required for influenza virus virion RNA synthesis. J Virol. 1992 Jul;66(7):4331–4338. doi: 10.1128/jvi.66.7.4331-4338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. X., Luytjes W., Enami M., Palese P. The polyadenylation signal of influenza virus RNA involves a stretch of uridines followed by the RNA duplex of the panhandle structure. J Virol. 1991 Jun;65(6):2861–2867. doi: 10.1128/jvi.65.6.2861-2867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., Chung J., Palese P. Alterations of the stalk of the influenza virus neuraminidase: deletions and insertions. Virus Res. 1993 Aug;29(2):141–153. doi: 10.1016/0168-1702(93)90055-r. [DOI] [PubMed] [Google Scholar]
- Luytjes W., Krystal M., Enami M., Parvin J. D., Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989 Dec 22;59(6):1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Jabbar M. A. Structural domains and organizational conformation involved in the sorting and transport of influenza virus transmembrane proteins. Annu Rev Microbiol. 1989;43:465–501. doi: 10.1146/annurev.mi.43.100189.002341. [DOI] [PubMed] [Google Scholar]
- Palese P. The genes of influenza virus. Cell. 1977 Jan;10(1):1–10. doi: 10.1016/0092-8674(77)90133-7. [DOI] [PubMed] [Google Scholar]
- Piccone M. E., Fernandez-Sesma A., Palese P. Mutational analysis of the influenza virus vRNA promoter. Virus Res. 1993 May;28(2):99–112. doi: 10.1016/0168-1702(93)90129-b. [DOI] [PubMed] [Google Scholar]
- Ryan M. D., Drew J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J. 1994 Feb 15;13(4):928–933. doi: 10.1002/j.1460-2075.1994.tb06337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. D., King A. M., Thomas G. P. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol. 1991 Nov;72(Pt 11):2727–2732. doi: 10.1099/0022-1317-72-11-2727. [DOI] [PubMed] [Google Scholar]
- Seto D. An improved method for sequencing double stranded plasmid DNA from minipreps using DMSO and modified template preparation. Nucleic Acids Res. 1990 Oct 11;18(19):5905–5906. doi: 10.1093/nar/18.19.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]