Abstract
The capsid proteins of papillomavirus self-assemble to form empty capsids or virus-like particles that appear quite similar to naturally occurring virions by conventional electron microscopy. To characterize such virus-like particles more fully, cryoelectron microscopy and image analysis techniques were used to generate three-dimensional reconstructions of capsids produced by vaccinia virus recombinants (V capsids) that expressed human papillomavirus type 1 L1 protein only or both L1 and L2 proteins. All V capsids had 72 pentameric capsomers arranged on a T = 7 icosahedral lattice. Each particle (approximately 60 nm in diameter) consisted of an approximately 2-nm-thick shell of protein with a radius of 22 nm with capsomers that extend approximately 6 nm from the shell. At a resolution of 3.5 nm, both V capsid structures appear identical to the capsid structure of native human papillomavirus type 1 (T. S. Baker, W. W. Newcomb, N. H. Olson, L. M. Cowsert, C. Olson, and J. C. Brown, Biophys. J. 60:1445-1456, 1991), thus implying that expressed and native capsids are structurally equivalent.
Full text
PDF
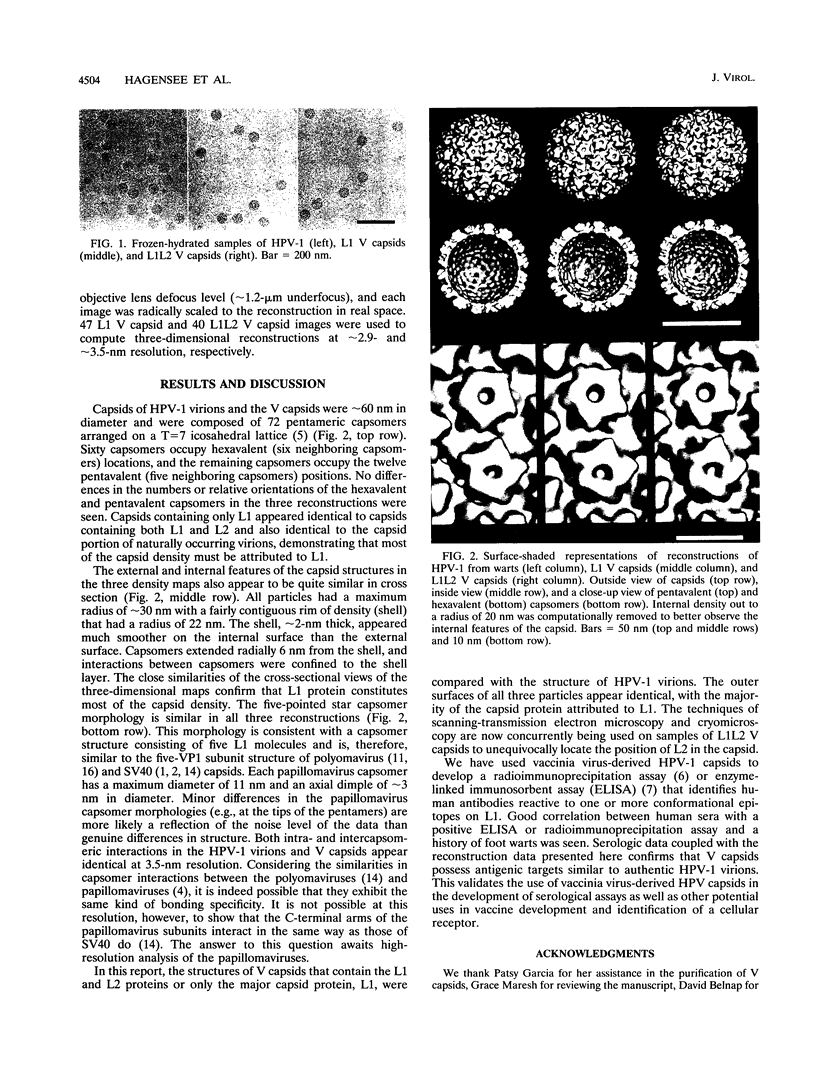

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. S., Drak J., Bina M. Reconstruction of the three-dimensional structure of simian virus 40 and visualization of the chromatin core. Proc Natl Acad Sci U S A. 1988 Jan;85(2):422–426. doi: 10.1073/pnas.85.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Drak J., Bina M. The capsid of small papova viruses contains 72 pentameric capsomeres: direct evidence from cryo-electron-microscopy of simian virus 40. Biophys J. 1989 Feb;55(2):243–253. doi: 10.1016/S0006-3495(89)82799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Newcomb W. W., Booy F. P., Brown J. C., Steven A. C. Three-dimensional structures of maturable and abortive capsids of equine herpesvirus 1 from cryoelectron microscopy. J Virol. 1990 Feb;64(2):563–573. doi: 10.1128/jvi.64.2.563-573.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Newcomb W. W., Olson N. H., Cowsert L. M., Olson C., Brown J. C. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991 Dec;60(6):1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. J., Hagensee M. B., Lee S. K., McKnight B., Koutsky L. A., Galloway D. A. Use of HPV 1 capsids produced by recombinant vaccinia viruses in an ELISA to detect serum antibodies in people with foot warts. Virology. 1994 Mar;199(2):284–291. doi: 10.1006/viro.1994.1126. [DOI] [PubMed] [Google Scholar]
- Carter J. J., Hagensee M., Taflin M. C., Lee S. K., Koutsky L. A., Galloway D. A. HPV-1 capsids expressed in vitro detect human serum antibodies associated with foot warts. Virology. 1993 Aug;195(2):456–462. doi: 10.1006/viro.1993.1396. [DOI] [PubMed] [Google Scholar]
- Cheng R. H., Reddy V. S., Olson N. H., Fisher A. J., Baker T. S., Johnson J. E. Functional implications of quasi-equivalence in a T = 3 icosahedral animal virus established by cryo-electron microscopy and X-ray crystallography. Structure. 1994 Apr 15;2(4):271–282. doi: 10.1016/s0969-2126(00)00029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M., Breitburd F., Croissant O., Orth G. Chromatin-like structures obtained after alkaline disruption of bovine and human papillomaviruses. J Virol. 1977 Mar;21(3):1205–1209. doi: 10.1128/jvi.21.3.1205-1209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J Virol. 1975 May;15(5):1239–1247. doi: 10.1128/jvi.15.5.1239-1247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. P., Griffith D. L., Rayment I., Murakami W. T., Caspar D. L. Inside polyomavirus at 25-A resolution. Nature. 1992 Feb 13;355(6361):652–654. doi: 10.1038/355652a0. [DOI] [PubMed] [Google Scholar]
- Hagensee M. E., Yaegashi N., Galloway D. A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993 Jan;67(1):315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R., Booy F., Cheng N., Lowy D. R., Schiller J. T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddington R. C., Yan Y., Moulai J., Sahli R., Benjamin T. L., Harrison S. C. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991 Nov 28;354(6351):278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- Orth G., Breitburd F., Favre M. Evidence for antigenic determinants shared by the structural polypeptides of (Shope) rabbit papillomavirus and human papillomavirus type 1. Virology. 1978 Dec;91(2):243–255. doi: 10.1016/0042-6822(78)90373-2. [DOI] [PubMed] [Google Scholar]
- Rayment I., Baker T. S., Caspar D. L., Murakami W. T. Polyoma virus capsid structure at 22.5 A resolution. Nature. 1982 Jan 14;295(5845):110–115. doi: 10.1038/295110a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. C., Bonnez W., Reichman R. C., Garcea R. L. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J Virol. 1993 Apr;67(4):1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salunke D. M., Caspar D. L., Garcea R. L. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986 Sep 12;46(6):895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- Yeager M., Dryden K. A., Olson N. H., Greenberg H. B., Baker T. S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J Cell Biol. 1990 Jun;110(6):2133–2144. doi: 10.1083/jcb.110.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Sun X. Y., Stenzel D. J., Frazer I. H. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991 Nov;185(1):251–257. doi: 10.1016/0042-6822(91)90772-4. [DOI] [PubMed] [Google Scholar]