Abstract
Murine coronaviruses such as mouse hepatitis virus (MHV) infect mouse cells via cellular receptors that are isoforms of biliary glycoprotein (Bgp) of the carcinoembryonic antigen gene family (G. S. Dveksler, C. W. Dieffenbach, C. B. Cardellichio, K. McCuaig, M. N. Pensiero, G.-S. Jiang, N. Beauchemin, and K. V. Holmes, J. Virol. 67:1-8, 1993). The Bgp isoforms are generated through alternative splicing of the mouse Bgp1 gene that has two allelic forms called MHVR (or mmCGM1), expressed in MHV-susceptible mouse strains, and mmCGM2, expressed in SJL/J mice, which are resistant to MHV. We here report the cloning and characterization of a new Bgp-related gene designated Bgp2. The Bgp2 cDNA allowed the prediction of a 271-amino-acid glycoprotein with two immunoglobulin domains, a transmembrane, and a putative cytoplasmic tail. There is considerable divergence in the amino acid sequences of the N-terminal domains of the proteins coded by the Bgp1 gene from that of the Bgp2-encoded protein. RNase protection assays and RNA PCR showed that Bgp2 was expressed in BALB/c kidney, colon, and brain tissue, in SJL/J colon and liver tissue, in BALB/c and CD1 spleen tissue, in C3H macrophages, and in mouse rectal carcinoma CMT-93 cells. When Bgp2-transfected hamster cells were challenged with MHV-A59, MHV-JHM, or MHV-3, the Bgp2-encoded protein served as a functional MHV receptor, although with a lower efficiency than that of the MHVR glycoprotein. The Bgp2-mediated virus infection could not be inhibited by monoclonal antibody CC1 that is specific for the N-terminal domain of MHVR. Although CMT-93 cells express both MHVR and Bgp2, infection with the three strains of MHV was blocked by pretreatment with monoclonal antibody CC1, suggesting that MHVR was the only functional receptor in these cells. Thus, a novel murine Bgp gene has been identified that can be coexpressed in inbred mice with the Bgp1 glycoproteins and that can serve as a receptor for MHV strains when expressed in transfected hamster cells.
Full text
PDF


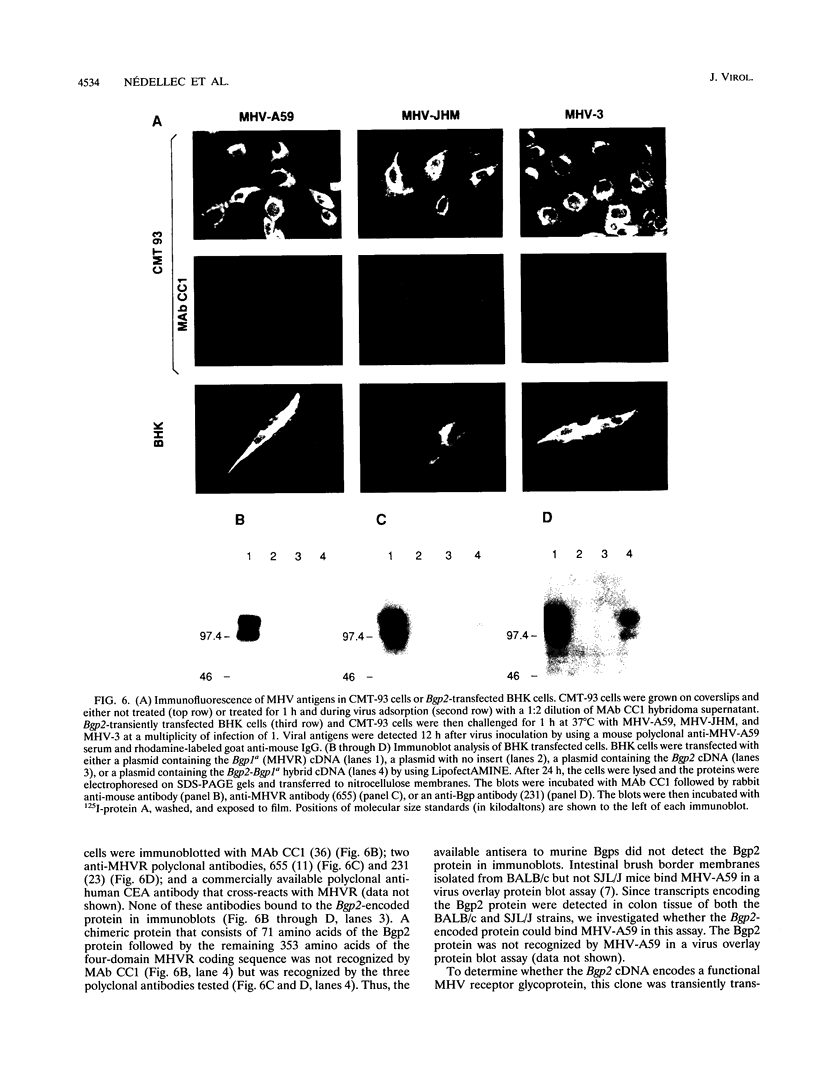



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afar D. E., Stanners C. P., Bell J. C. Tyrosine phosphorylation of biliary glycoprotein, a cell adhesion molecule related to carcinoembryonic antigen. Biochim Biophys Acta. 1992 Feb 19;1134(1):46–52. doi: 10.1016/0167-4889(92)90026-8. [DOI] [PubMed] [Google Scholar]
- Barnett T. R., Drake L., Pickle W., 2nd Human biliary glycoprotein gene: characterization of a family of novel alternatively spliced RNAs and their expressed proteins. Mol Cell Biol. 1993 Feb;13(2):1273–1282. doi: 10.1128/mcb.13.2.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett T. R., Kretschmer A., Austen D. A., Goebel S. J., Hart J. T., Elting J. J., Kamarck M. E. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989 Feb;108(2):267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin N., Benchimol S., Cournoyer D., Fuks A., Stanners C. P. Isolation and characterization of full-length functional cDNA clones for human carcinoembryonic antigen. Mol Cell Biol. 1987 Sep;7(9):3221–3230. doi: 10.1128/mcb.7.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Weismiller D. G., Holmes K. V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987 Jan;61(1):185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattain M. G., Strobel-Stevens J., Fine D., Webb M., Sarrif A. M. Establishment of mouse colonic carcinoma cell lines with different metastatic properties. Cancer Res. 1980 Jul;40(7):2142–2146. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dveksler G. S., Pensiero M. N., Cardellichio C. B., Williams R. K., Jiang G. S., Holmes K. V., Dieffenbach C. W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991 Dec;65(12):6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dveksler G. S., Pensiero M. N., Dieffenbach C. W., Cardellichio C. B., Basile A. A., Elia P. E., Holmes K. V. Mouse hepatitis virus strain A59 and blocking antireceptor monoclonal antibody bind to the N-terminal domain of cellular receptor. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1716–1720. doi: 10.1073/pnas.90.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feracci H., Connolly T. P., Margolis R. N., Hubbard A. L. The establishment of hepatocyte cell surface polarity during fetal liver development. Dev Biol. 1987 Sep;123(1):73–84. doi: 10.1016/0012-1606(87)90429-5. [DOI] [PubMed] [Google Scholar]
- Huang J. Q., Turbide C., Daniels E., Jothy S., Beauchemin N. Spatiotemporal expression of murine carcinoembryonic antigen (CEA) gene family members during mouse embryogenesis. Development. 1990 Oct;110(2):573–588. doi: 10.1242/dev.110.2.573. [DOI] [PubMed] [Google Scholar]
- Kuroki M., Arakawa F., Matsuo Y., Oikawa S., Nakazato H., Matsuoka Y. Three novel molecular forms of biliary glycoprotein deduced from cDNA clones from a human leukocyte library. Biochem Biophys Res Commun. 1991 Apr 30;176(2):578–585. doi: 10.1016/s0006-291x(05)80223-2. [DOI] [PubMed] [Google Scholar]
- Leusch H. G., Drzeniek Z., Markos-Pusztai Z., Wagener C. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic alpha-glycosides of mannose. Infect Immun. 1991 Jun;59(6):2051–2057. doi: 10.1128/iai.59.6.2051-2057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. H., Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989 Aug 25;264(24):14408–14414. [PubMed] [Google Scholar]
- Margolis R. N., Taylor S. I., Seminara D., Hubbard A. L. Identification of pp120, an endogenous substrate for the hepatocyte insulin receptor tyrosine kinase, as an integral membrane glycoprotein of the bile canalicular domain. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7256–7259. doi: 10.1073/pnas.85.19.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuaig K., Rosenberg M., Nédellec P., Turbide C., Beauchemin N. Expression of the Bgp gene and characterization of mouse colon biliary glycoprotein isoforms. Gene. 1993 May 30;127(2):173–183. doi: 10.1016/0378-1119(93)90716-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuaig K., Turbide C., Beauchemin N. mmCGM1a: a mouse carcinoembryonic antigen gene family member, generated by alternative splicing, functions as an adhesion molecule. Cell Growth Differ. 1992 Mar;3(3):165–174. [PubMed] [Google Scholar]
- Najjar S. M., Accili D., Philippe N., Jernberg J., Margolis R., Taylor S. I. pp120/ecto-ATPase, an endogenous substrate of the insulin receptor tyrosine kinase, is expressed as two variably spliced isoforms. J Biol Chem. 1993 Jan 15;268(2):1201–1206. [PubMed] [Google Scholar]
- Obrink B. C-CAM (cell-CAM 105)--a member of the growing immunoglobulin superfamily of cell adhesion proteins. Bioessays. 1991 May;13(5):227–234. doi: 10.1002/bies.950130505. [DOI] [PubMed] [Google Scholar]
- Ocklind C., Obrink B. Intercellular adhesion of rat hepatocytes. Identification of a cell surface glycoprotein involved in the initial adhesion process. J Biol Chem. 1982 Jun 25;257(12):6788–6795. [PubMed] [Google Scholar]
- Oikawa S., Inuzuka C., Kuroki M., Arakawa F., Matsuoka Y., Kosaki G., Nakazato H. A specific heterotypic cell adhesion activity between members of carcinoembryonic antigen family, W272 and NCA, is mediated by N-domains. J Biol Chem. 1991 May 5;266(13):7995–8001. [PubMed] [Google Scholar]
- Robbins J., Robbins P. F., Kozak C. A., Callahan R. The mouse biliary glycoprotein gene (Bgp): partial nucleotide sequence, expression, and chromosomal assignment. Genomics. 1991 Jul;10(3):583–587. doi: 10.1016/0888-7543(91)90439-l. [DOI] [PubMed] [Google Scholar]
- Rojas M., Fuks A., Stanners C. P. Biliary glycoprotein, a member of the immunoglobulin supergene family, functions in vitro as a Ca2(+)-dependent intercellular adhesion molecule. Cell Growth Differ. 1990 Nov;1(11):527–533. [PubMed] [Google Scholar]
- Rosenberg M., Nédellec P., Jothy S., Fleiszer D., Turbide C., Beauchemin N. The expression of mouse biliary glycoprotein, a carcinoembryonic antigen-related gene, is down-regulated in malignant mouse tissues. Cancer Res. 1993 Oct 15;53(20):4938–4945. [PubMed] [Google Scholar]
- Rudert F., Saunders A. M., Rebstock S., Thompson J. A., Zimmermann W. Characterization of murine carcinoembryonic antigen gene family members. Mamm Genome. 1992;3(5):262–273. doi: 10.1007/BF00292154. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrewe H., Thompson J., Bona M., Hefta L. J., Maruya A., Hassauer M., Shively J. E., von Kleist S., Zimmermann W. Cloning of the complete gene for carcinoembryonic antigen: analysis of its promoter indicates a region conveying cell type-specific expression. Mol Cell Biol. 1990 Jun;10(6):2738–2748. doi: 10.1128/mcb.10.6.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel C. J., Suchy F. J., Ananthanarayanan M., Perlmutter D. H. The rat liver ecto-ATPase is also a canalicular bile acid transport protein. J Biol Chem. 1993 Jan 25;268(3):2083–2091. [PubMed] [Google Scholar]
- Smith A. L., Cardellichio C. B., Winograd D. F., de Souza M. S., Barthold S. W., Holmes K. V. Monoclonal antibody to the receptor for murine coronavirus MHV-A59 inhibits viral replication in vivo. J Infect Dis. 1991 Apr;163(4):879–882. doi: 10.1093/infdis/163.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman L. S., Takemoto K. K. Enhanced growth of a murine coronavirus in transformed mouse cells. Infect Immun. 1972 Oct;6(4):501–507. doi: 10.1128/iai.6.4.501-507.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svalander P. C., Odin P., Nilsson B. O., Obrink B. Expression of cellCAM-105 in the apical surface of rat uterine epithelium is controlled by ovarian steroid hormones. J Reprod Fertil. 1990 Jan;88(1):213–221. doi: 10.1530/jrf.0.0880213. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Grunert F., Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5(5):344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- Turbide C., Rojas M., Stanners C. P., Beauchemin N. A mouse carcinoembryonic antigen gene family member is a calcium-dependent cell adhesion molecule. J Biol Chem. 1991 Jan 5;266(1):309–315. [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Willcocks T. C., Craig I. W. Characterization of the genomic organization of human carcinoembryonic antigen (CEA): comparison with other family members and sequence analysis of 5' controlling region. Genomics. 1990 Nov;8(3):492–500. doi: 10.1016/0888-7543(90)90036-t. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Holmes K. V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Snyder S. W., Frana M. F., Holmes K. V. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J Virol. 1990 Aug;64(8):3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori K., Asanaka M., Stohlman S. A., Lai M. M. A spike protein-dependent cellular factor other than the viral receptor is required for mouse hepatitis virus entry. Virology. 1993 Sep;196(1):45–56. doi: 10.1006/viro.1993.1453. [DOI] [PubMed] [Google Scholar]
- Yokomori K., Lai M. M. Mouse hepatitis virus utilizes two carcinoembryonic antigens as alternative receptors. J Virol. 1992 Oct;66(10):6194–6199. doi: 10.1128/jvi.66.10.6194-6199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori K., Lai M. M. The receptor for mouse hepatitis virus in the resistant mouse strain SJL is functional: implications for the requirement of a second factor for viral infection. J Virol. 1992 Dec;66(12):6931–6938. doi: 10.1128/jvi.66.12.6931-6938.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Fuks A., Alcaraz G., Bolling T. J., Stanners C. P. Homophilic adhesion between Ig superfamily carcinoembryonic antigen molecules involves double reciprocal bonds. J Cell Biol. 1993 Aug;122(4):951–960. doi: 10.1083/jcb.122.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]