Abstract
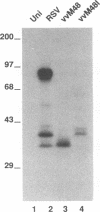
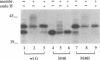
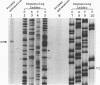
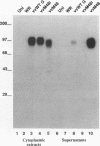
Respiratory syncytial (RS) virus synthesizes two mature forms of its attachment glycoprotein G: an anchored type II integral membrane form and a smaller form that is secreted into the medium. Here we demonstrate that these two forms are synthesized as distinct primary translation products of a single species of G protein mRNA by initiation at either of two different AUGs. Mutant cDNAs which eliminated one of the other of the two AUG codons near the 5' end of the G gene open reading frame were constructed. Analysis of the proteins synthesized from these cDNAs, either by translation of transcripts in a cell-free system or in cells infected with recombinant vaccinia viruses containing either one of the mutant cDNAs, showed that elimination of either the first or the second of these AUG codons abrogated the synthesis of the membrane-anchored or the secreted form of the protein, respectively. Additionally, two unglycosylated forms of G protein which comigrated with the unglycosylated G proteins expressed by these recombinant viruses were detected in RS virus-infected cells. Since the second AUG encodes a methionine residue that lies near the middle of the signal/anchor domain, initiation at this codon resulted in a protein with a hydrophobic amino terminus. This form of the glycoprotein was efficiently secreted from cells infected with the vaccinia virus recombinant, and the amino-terminal sequence of this protein was identical to that of G protein secreted from RS virus-infected cells. Our results demonstrate that the secreted form of RS virus G protein is produced by initiation at the second AUG codon of the G open reading frame, followed by proteolytic removal of the signal/anchor domain.
Full text
PDF




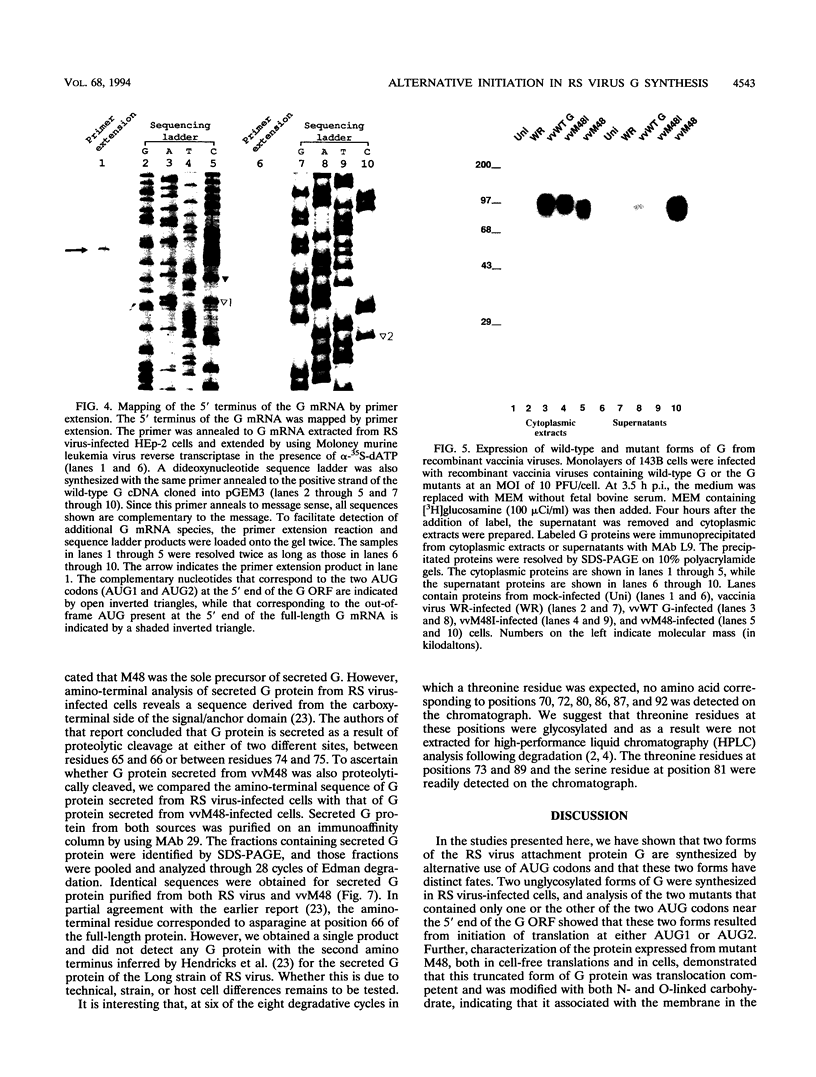
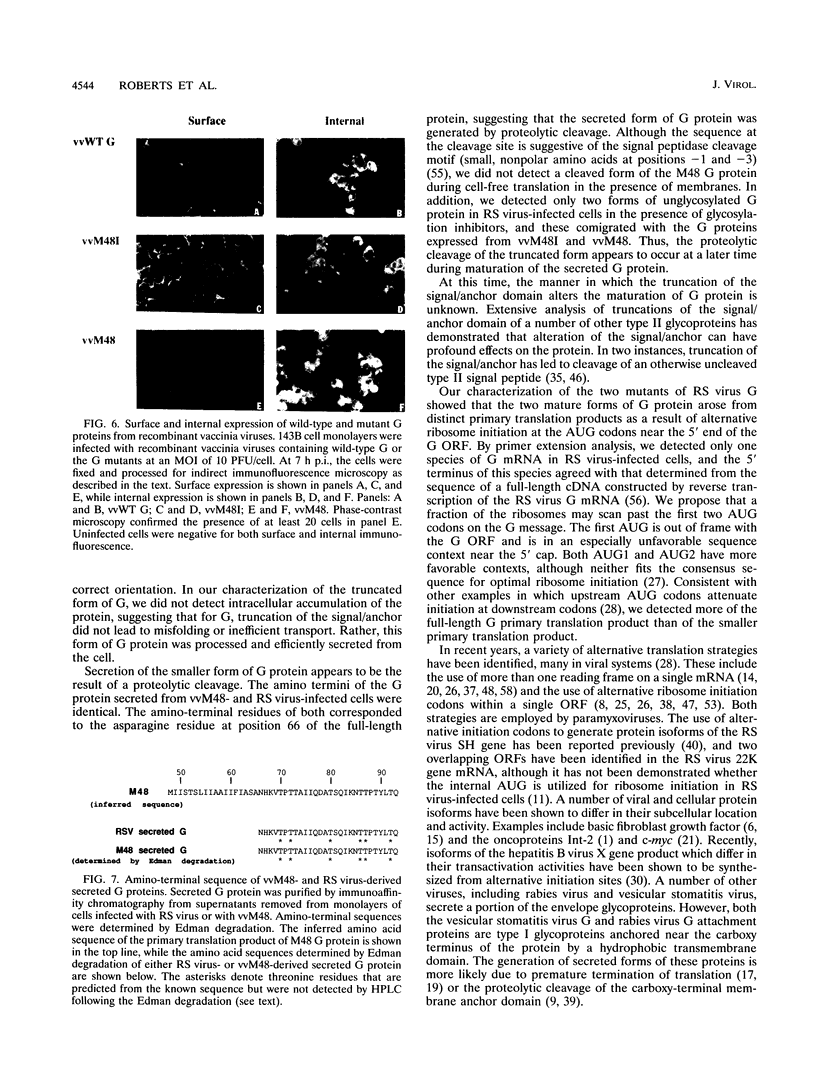


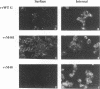
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Alansari H., Potgieter L. N. Nucleotide sequence analysis of the ovine respiratory syncytial virus G glycoprotein gene. Virology. 1993 Oct;196(2):873–877. doi: 10.1006/viro.1993.1549. [DOI] [PubMed] [Google Scholar]
- Ball L. A., Young K. K., Anderson K., Collins P. L., Wertz G. W. Expression of the major glycoprotein G of human respiratory syncytial virus from recombinant vaccinia virus vectors. Proc Natl Acad Sci U S A. 1986 Jan;83(2):246–250. doi: 10.1073/pnas.83.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugler B., Amalric F., Prats H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol Cell Biol. 1991 Jan;11(1):573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane P. A., Matthews D. A., Pringle C. R. Identification of variable domains of the attachment (G) protein of subgroup A respiratory syncytial viruses. J Gen Virol. 1991 Sep;72(Pt 9):2091–2096. doi: 10.1099/0022-1317-72-9-2091. [DOI] [PubMed] [Google Scholar]
- Chan W. K., Penaranda M. E., Crawford S. E., Estes M. K. Two glycoproteins are produced from the rotavirus neutralization gene. Virology. 1986 Jun;151(2):243–252. doi: 10.1016/0042-6822(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Ariel N., Huang A. S. Membrane anchors of vesicular stomatitis virus: characterization and incorporation into virions. J Virol. 1988 Aug;62(8):2552–2556. doi: 10.1128/jvi.62.8.2552-2556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hill M. G., Johnson P. R. The two open reading frames of the 22K mRNA of human respiratory syncytial virus: sequence comparison of antigenic subgroups A and B and expression in vitro. J Gen Virol. 1990 Dec;71(Pt 12):3015–3020. doi: 10.1099/0022-1317-71-12-3015. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Identification of a tenth mRNA of respiratory syncytial virus and assignment of polypeptides to the 10 viral genes. J Virol. 1984 Feb;49(2):572–578. doi: 10.1128/jvi.49.2.572-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Mottet G. Oligomerization and post-translational processing of glycoprotein G of human respiratory syncytial virus: altered O-glycosylation in the presence of brefeldin A. J Gen Virol. 1992 Apr;73(Pt 4):849–863. doi: 10.1099/0022-1317-73-4-849. [DOI] [PubMed] [Google Scholar]
- Collins P. L. O glycosylation of glycoprotein G of human respiratory syncytial virus is specified within the divergent ectodomain. J Virol. 1990 Aug;64(8):4007–4012. doi: 10.1128/jvi.64.8.4007-4012.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 1988 Jan;7(1):245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeve L., Garreis-Wabnitz C., Zauke M., Breindl M., Kruppa J. The soluble glycoprotein of vesicular stomatitis virus is formed during or shortly after the translation process. J Virol. 1986 Mar;57(3):968–975. doi: 10.1128/jvi.57.3.968-975.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber C., Levine S. Respiratory syncytial virus polypeptides. IV. The oligosaccharides of the glycoproteins. J Gen Virol. 1985 Mar;66(Pt 3):417–432. doi: 10.1099/0022-1317-66-3-417. [DOI] [PubMed] [Google Scholar]
- Grünberg J., Kruppa A., Paschen P., Kruppa J. Intracellular formation of two soluble glycoproteins in BHK cells infected with vesicular stomatitis virus serotype New Jersey. Virology. 1991 Feb;180(2):678–686. doi: 10.1016/0042-6822(91)90081-l. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Patwardhan S. ACG, the initiator codon for a Sendai virus protein. J Biol Chem. 1988 Jun 25;263(18):8553–8556. [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Hendricks D. A., Baradaran K., McIntosh K., Patterson J. L. Appearance of a soluble form of the G protein of respiratory syncytial virus in fluids of infected cells. J Gen Virol. 1987 Jun;68(Pt 6):1705–1714. doi: 10.1099/0022-1317-68-6-1705. [DOI] [PubMed] [Google Scholar]
- Hendricks D. A., McIntosh K., Patterson J. L. Further characterization of the soluble form of the G glycoprotein of respiratory syncytial virus. J Virol. 1988 Jul;62(7):2228–2233. doi: 10.1128/jvi.62.7.2228-2233.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. R., Spriggs M. K., Olmsted R. A., Collins P. L. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakach L. T., Wasmoen T. L., Collett M. S. Rift Valley fever virus M segment: use of recombinant vaccinia viruses to study Phlebovirus gene expression. J Virol. 1988 Mar;62(3):826–833. doi: 10.1128/jvi.62.3.826-833.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee L., Lucito R., Aufiero B., Schneider R. J. Alternate translation initiation on hepatitis B virus X mRNA produces multiple polypeptides that differentially transactivate class II and III promoters. J Virol. 1992 Jul;66(7):4382–4389. doi: 10.1128/jvi.66.7.4382-4389.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. M. Role of oligosaccharides in the structure and function of respiratory syncytial virus glycoproteins. Virology. 1988 Jun;164(2):458–466. doi: 10.1016/0042-6822(88)90560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch R. A., Anderson K., Wertz G. W. Nucleotide sequence analysis and expression from recombinant vectors demonstrate that the attachment protein G of bovine respiratory syncytial virus is distinct from that of human respiratory syncytial virus. J Virol. 1990 Nov;64(11):5559–5569. doi: 10.1128/jvi.64.11.5559-5569.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. The membrane-spanning segment of invariant chain (I gamma) contains a potentially cleavable signal sequence. Cell. 1986 Sep 26;46(7):1103–1112. doi: 10.1016/0092-8674(86)90710-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallipeddi S. K., Samal S. K. Sequence variability of the glycoprotein gene of bovine respiratory syncytial virus. J Gen Virol. 1993 Sep;74(Pt 9):2001–2004. doi: 10.1099/0022-1317-74-9-2001. [DOI] [PubMed] [Google Scholar]
- McGinnes L., McQuain C., Morrison T. The P protein and the nonstructural 38K and 29K proteins of Newcastle disease virus are derived from the same open reading frame. Virology. 1988 May;164(1):256–264. doi: 10.1016/0042-6822(88)90643-5. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Iwatani Y., Kawai A. Shedding of Gs protein (a soluble form of the viral glycoprotein) by the rabies virus-infected BHK-21 cells. Virology. 1993 Aug;195(2):541–549. doi: 10.1006/viro.1993.1405. [DOI] [PubMed] [Google Scholar]
- Olmsted R. A., Collins P. L. The 1A protein of respiratory syncytial virus is an integral membrane protein present as multiple, structurally distinct species. J Virol. 1989 May;63(5):2019–2029. doi: 10.1128/jvi.63.5.2019-2029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R. A., Murphy B. R., Lawrence L. A., Elango N., Moss B., Collins P. L. Processing, surface expression, and immunogenicity of carboxy-terminally truncated mutants of G protein of human respiratory syncytial virus. J Virol. 1989 Jan;63(1):411–420. doi: 10.1128/jvi.63.1.411-420.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik A. K., Ball L. A., LeGrone A. W., Wertz G. W. Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell. 1992 Jun 12;69(6):1011–1020. doi: 10.1016/0092-8674(92)90619-n. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Satake M., Coligan J. E., Elango N., Norrby E., Venkatesan S. Respiratory syncytial virus envelope glycoprotein (G) has a novel structure. Nucleic Acids Res. 1985 Nov 11;13(21):7795–7812. doi: 10.1093/nar/13.21.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. R., Spiess M. Deletion of the amino-terminal domain of asialoglycoprotein receptor H1 allows cleavage of the internal signal sequence. J Biol Chem. 1988 Nov 15;263(32):16886–16891. [PubMed] [Google Scholar]
- Sedman S. A., Mertz J. E. Mechanisms of synthesis of virion proteins from the functionally bigenic late mRNAs of simian virus 40. J Virol. 1988 Mar;62(3):954–961. doi: 10.1128/jvi.62.3.954-961.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiropoulou C. F., Nichol S. T. A small highly basic protein is encoded in overlapping frame within the P gene of vesicular stomatitis virus. J Virol. 1993 Jun;67(6):3103–3110. doi: 10.1128/jvi.67.6.3103-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Ball L. A., Young K. K., Furze J., Wertz G. W. Human respiratory syncytial virus glycoprotein G expressed from a recombinant vaccinia virus vector protects mice against live-virus challenge. J Virol. 1986 Nov;60(2):607–613. doi: 10.1128/jvi.60.2.607-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J., Taylor G., Ball L. A., Anderson K., Young K. K., King A. M., Wertz G. W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987 Dec;61(12):3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullender W. M., Mufson M. A., Anderson L. J., Wertz G. W. Genetic diversity of the attachment protein of subgroup B respiratory syncytial viruses. J Virol. 1991 Oct;65(10):5425–5434. doi: 10.1128/jvi.65.10.5425-5434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzich J. A., Kakach L. T., Collett M. S. Expression strategy of a phlebovirus: biogenesis of proteins from the Rift Valley fever virus M segment. J Virol. 1990 Apr;64(4):1549–1555. doi: 10.1128/jvi.64.4.1549-1555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaya S., Elango N., Zavala F., Moss B. Transport to the cell surface of a peptide sequence attached to the truncated C terminus of an N-terminally anchored integral membrane protein. Mol Cell Biol. 1988 Apr;8(4):1709–1714. doi: 10.1128/mcb.8.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Collins P. L., Huang Y., Gruber C., Levine S., Ball L. A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Krieger M., Ball L. A. Structure and cell surface maturation of the attachment glycoprotein of human respiratory syncytial virus in a cell line deficient in O glycosylation. J Virol. 1989 Nov;63(11):4767–4776. doi: 10.1128/jvi.63.11.4767-4776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. A., Lamb R. A. Effect of mutations and deletions in a bicistronic mRNA on the synthesis of influenza B virus NB and NA glycoproteins. J Virol. 1989 Jan;63(1):28–35. doi: 10.1128/jvi.63.1.28-35.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. The signal peptide. J Membr Biol. 1990 May;115(3):195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]