Abstract
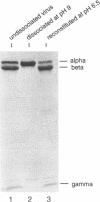
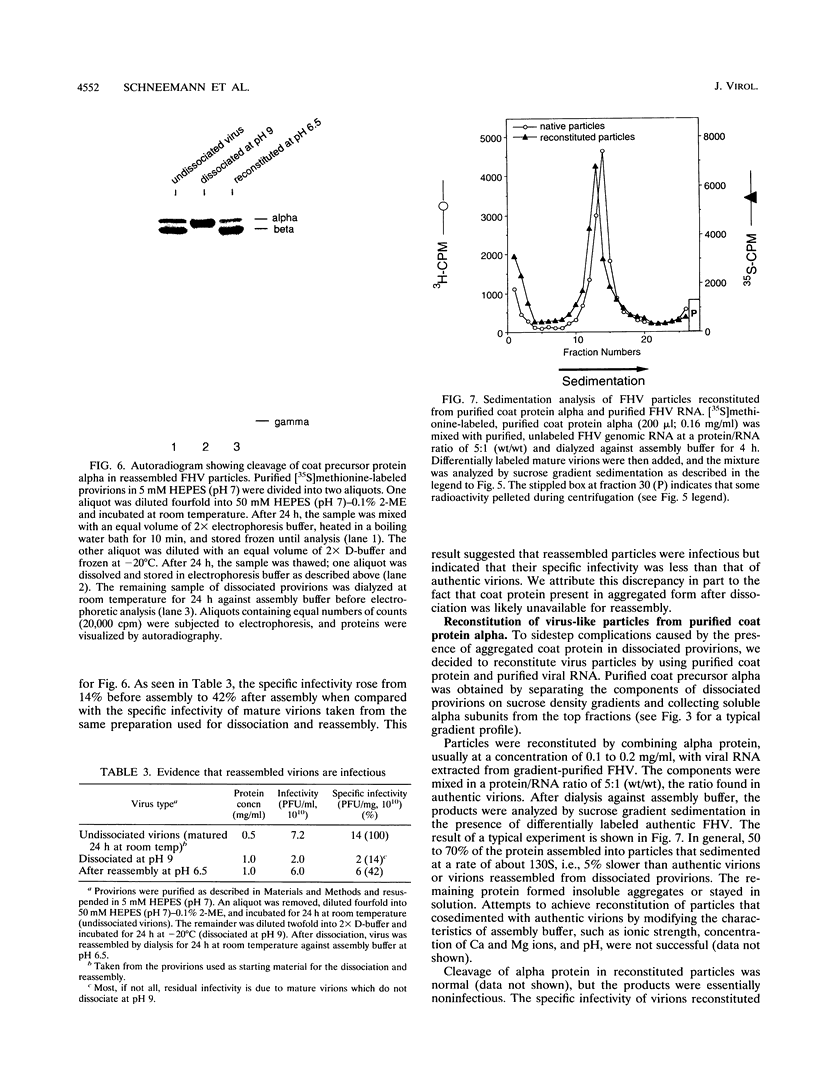
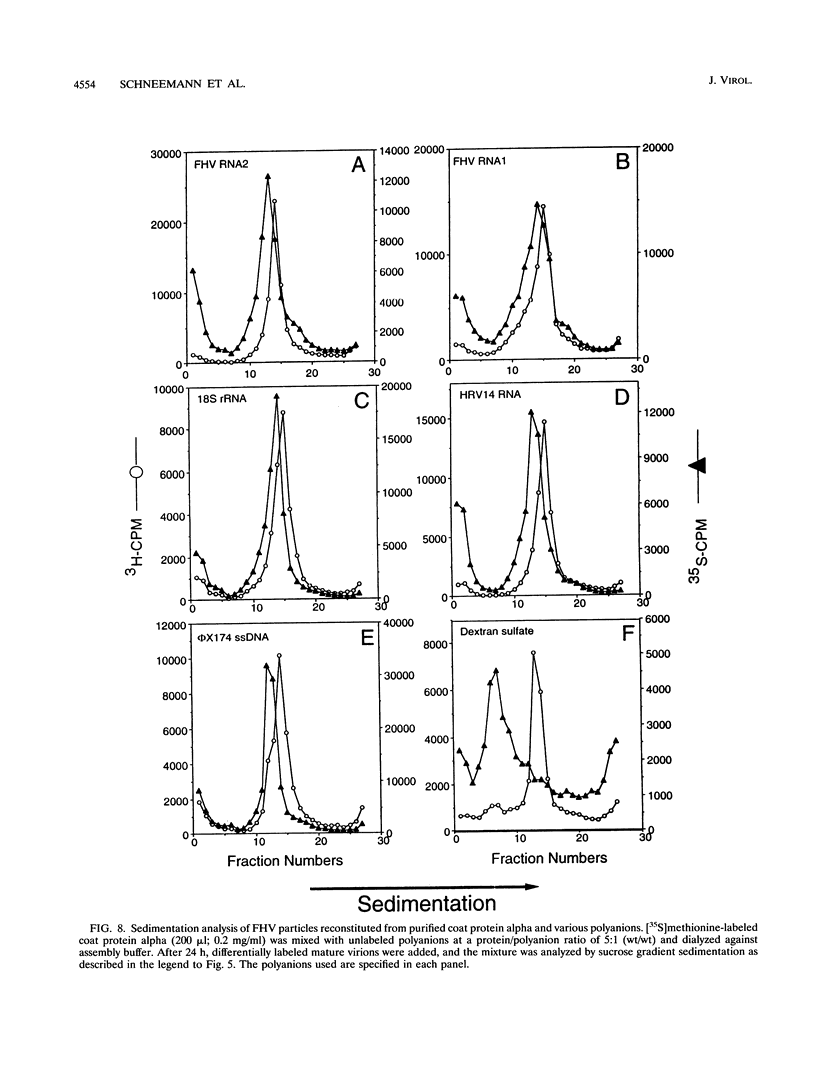
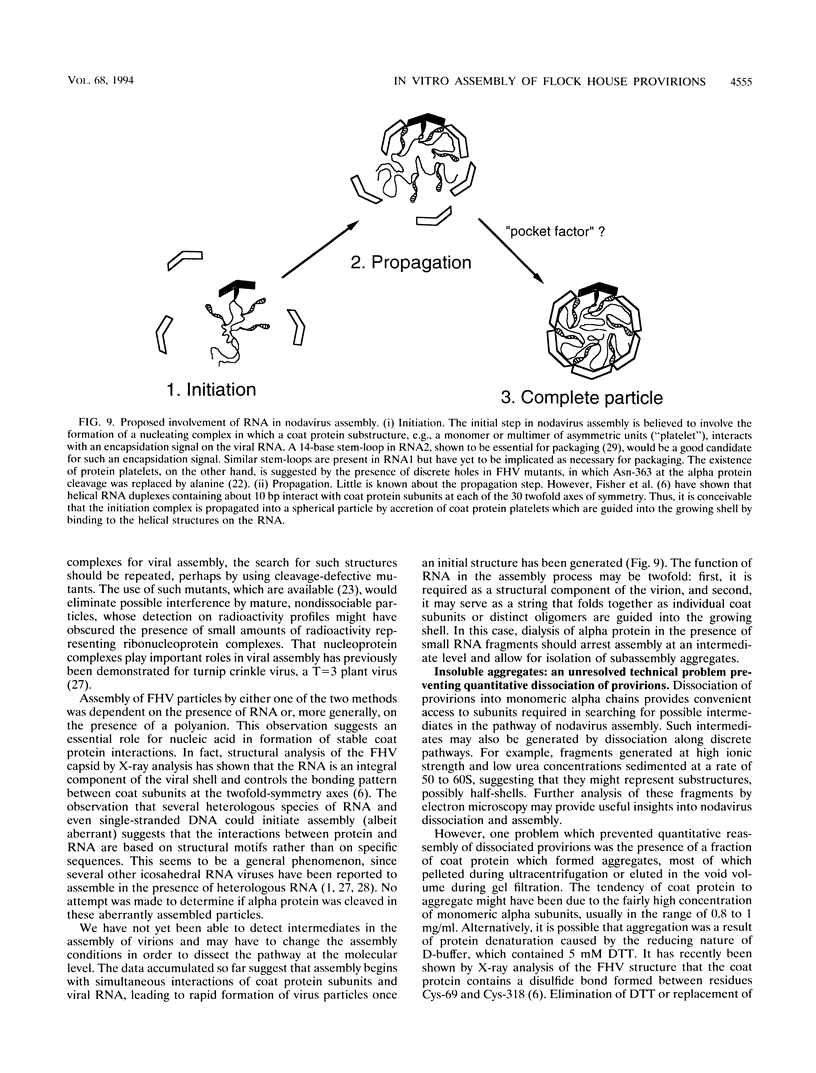
Assembly of Flock House virus in infected Drosophila cells proceeds through an intermediate, the provirion, which lacks infectivity until the coat precursor protein, alpha, undergoes a spontaneous "maturation" cleavage (A. Schneemann, W. Zhong, T. M. Gallagher, and R. R. Rueckert, J. Virol 6:6728, 1992). We describe here methods for purifying provirions in a state which permitted dissociation and reassembly. Dissociation, to monomeric alpha protein and free RNA, was accomplished by freezing at pH 9.0 in the presence of 0.5 M salt and 0.1 M urea. When dialyzed at low ionic strength and pH 6.5, the dissociation products reassembled spontaneously to form homogeneous provirions with a normal complement of RNA as judged by cosedimentation with authentic virions and by ability to undergo maturation cleavage with acquisition of substantial, though subnormal, infectivity. Reconstitution experiments, i.e., remixing components after separating RNA from capsid protein, generated abnormal particles, suggesting the presence in the unfractionated dissociation products of an unidentified "nucleating" component.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft J. B. The self-assembly of spherical plant viruses. Adv Virus Res. 1970;16:99–134. doi: 10.1016/s0065-3527(08)60022-6. [DOI] [PubMed] [Google Scholar]
- Dasgupta R., Ghosh A., Dasmahapatra B., Guarino L. A., Kaesberg P. Primary and secondary structure of black beetle virus RNA2, the genomic messenger for BBV coat protein precursor. Nucleic Acids Res. 1984 Sep 25;12(18):7215–7223. doi: 10.1093/nar/12.18.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta R., Sgro J. Y. Nucleotide sequences of three Nodavirus RNA2's: the messengers for their coat protein precursors. Nucleic Acids Res. 1989 Sep 25;17(18):7525–7526. doi: 10.1093/nar/17.18.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasmahapatra B., Dasgupta R., Ghosh A., Kaesberg P. Structure of the black beetle virus genome and its functional implications. J Mol Biol. 1985 Mar 20;182(2):183–189. doi: 10.1016/0022-2836(85)90337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasmahapatra B., Dasgupta R., Saunders K., Selling B., Gallagher T., Kaesberg P. Infectious RNA derived by transcription from cloned cDNA copies of the genomic RNA of an insect virus. Proc Natl Acad Sci U S A. 1986 Jan;83(1):63–66. doi: 10.1073/pnas.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. J., Johnson J. E. Ordered duplex RNA controls capsid architecture in an icosahedral animal virus. Nature. 1993 Jan 14;361(6408):176–179. doi: 10.1038/361176a0. [DOI] [PubMed] [Google Scholar]
- Friesen P. D., Rueckert R. R. Black beetle virus: messenger for protein B is a subgenomic viral RNA. J Virol. 1982 Jun;42(3):986–995. doi: 10.1128/jvi.42.3.986-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P. D., Rueckert R. R. Early and late functions in a bipartite RNA virus: evidence for translational control by competition between viral mRNAs. J Virol. 1984 Jan;49(1):116–124. doi: 10.1128/jvi.49.1.116-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P. D., Rueckert R. R. Synthesis of Black Beetle Virus Proteins in Cultured Drosophila Cells: Differential Expression of RNAs 1 and 2. J Virol. 1981 Mar;37(3):876–886. doi: 10.1128/jvi.37.3.876-886.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. M., Friesen P. D., Rueckert R. R. Autonomous replication and expression of RNA 1 from black beetle virus. J Virol. 1983 May;46(2):481–489. doi: 10.1128/jvi.46.2.481-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. M., Rueckert R. R. Assembly-dependent maturation cleavage in provirions of a small icosahedral insect ribovirus. J Virol. 1988 Sep;62(9):3399–3406. doi: 10.1128/jvi.62.9.3399-3406.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Ghosh A., Dasmahapatra B., Dasgupta R., Kaesberg P. Sequence of the black beetle virus subgenomic RNA and its location in the viral genome. Virology. 1984 Nov;139(1):199–203. doi: 10.1016/0042-6822(84)90342-8. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Kaesberg P. Isolation and Characterization of an RNA-Dependent RNA Polymerase from Black Beetle Virus-Infected Drosophila melanogaster Cells. J Virol. 1981 Nov;40(2):379–386. doi: 10.1128/jvi.40.2.379-386.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosur M. V., Schmidt T., Tucker R. C., Johnson J. E., Gallagher T. M., Selling B. H., Rueckert R. R. Structure of an insect virus at 3.0 A resolution. Proteins. 1987;2(3):167–176. doi: 10.1002/prot.340020302. [DOI] [PubMed] [Google Scholar]
- Kallenbach N. R. Theory of thermal transitions in low molecular weight RNA chains. J Mol Biol. 1968 Nov 14;37(3):445–466. doi: 10.1016/0022-2836(68)90114-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F. Further physicochemical characterization of Nodamura virus. Evidence that the divided genome occurs in a single component. J Gen Virol. 1978 Jan;38(1):83–95. doi: 10.1099/0022-1317-38-1-83. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER I. DIFFERENTIATION OF LARVAL DROSOPHILA EYE-ANTENNAL DISCS IN VITRO. J Exp Zool. 1964 Jun;156:91–103. doi: 10.1002/jez.1401560107. [DOI] [PubMed] [Google Scholar]
- Saunders K., Kaesberg P. Template-dependent RNA polymerase from black beetle virus-infected Drosophila melanogaster cells. Virology. 1985 Dec;147(2):373–381. doi: 10.1016/0042-6822(85)90139-4. [DOI] [PubMed] [Google Scholar]
- Schneemann A., Dasgupta R., Johnson J. E., Rueckert R. R. Use of recombinant baculoviruses in synthesis of morphologically distinct viruslike particles of flock house virus, a nodavirus. J Virol. 1993 May;67(5):2756–2763. doi: 10.1128/jvi.67.5.2756-2763.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneemann A., Zhong W., Gallagher T. M., Rueckert R. R. Maturation cleavage required for infectivity of a nodavirus. J Virol. 1992 Nov;66(11):6728–6734. doi: 10.1128/jvi.66.11.6728-6734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Selling B. H., Rueckert R. R. Plaque assay for black beetle virus. J Virol. 1984 Jul;51(1):251–253. doi: 10.1128/jvi.51.1.251-253.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Stockley P. G., Harrison S. C. Structure and assembly of turnip crinkle virus. II. Mechanism of reassembly in vitro. J Mol Biol. 1986 Oct 20;191(4):639–658. doi: 10.1016/0022-2836(86)90451-1. [DOI] [PubMed] [Google Scholar]
- Wengler G., Boege U., Wengler G., Bischoff H., Wahn K. The core protein of the alphavirus Sindbis virus assembles into core-like nucleoproteins with the viral genome RNA and with other single-stranded nucleic acids in vitro. Virology. 1982 Apr 30;118(2):401–410. doi: 10.1016/0042-6822(82)90359-2. [DOI] [PubMed] [Google Scholar]
- Zhong W., Dasgupta R., Rueckert R. Evidence that the packaging signal for nodaviral RNA2 is a bulged stem-loop. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11146–11150. doi: 10.1073/pnas.89.23.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]