Abstract
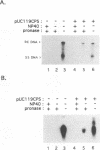
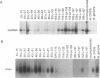
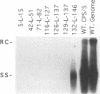
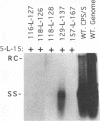
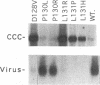
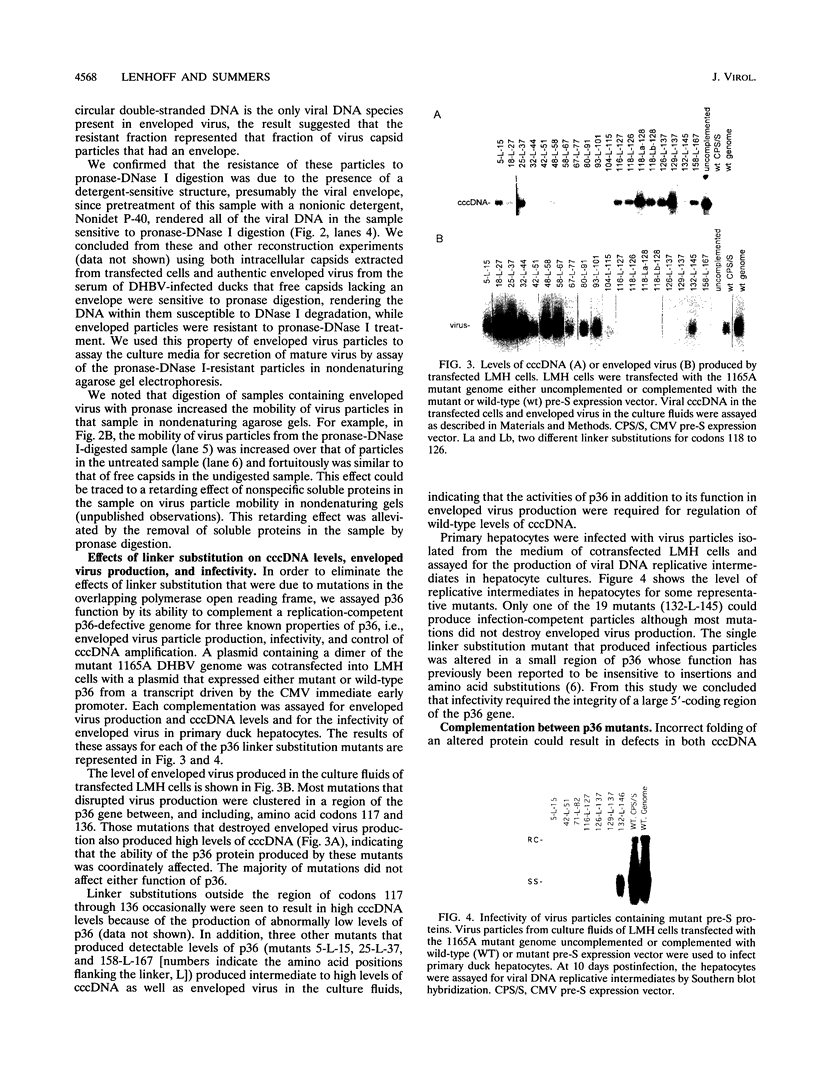
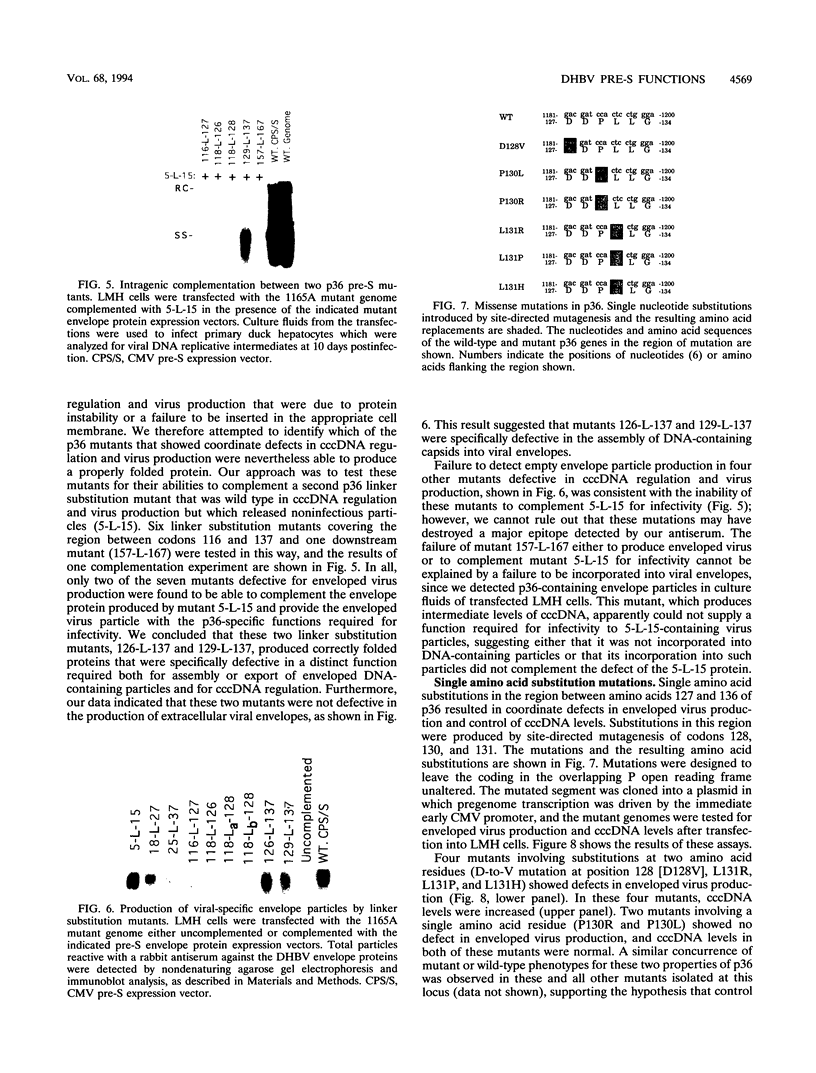
We have used linker scanning and site-directed mutagenesis in an attempt to distinguish among the known functions of the duck hepatitis B virus large envelope protein, p36. We found that linker-encoded amino acid substitutions in at least one region of the pre-S envelope protein p36 produced defects in both the production of enveloped virus and the regulation of covalently closed circular DNA (cccDNA) synthesis. Most linker substitutions, typically in the 5' two-thirds of the pre-S region of the p36 gene did not affect either cccDNA regulation or enveloped virus production but did destroy the infection competence of the enveloped particles produced. Single amino acid substitutions of residues 128 and 131 demonstrated a similar correlation between defects in the ability of p36 to support enveloped virus production and to control cccDNA levels. We concluded from these studies that virus production and cccDNA regulation probably require a common activity of p36.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büscher M., Reiser W., Will H., Schaller H. Transcripts and the putative RNA pregenome of duck hepatitis B virus: implications for reverse transcription. Cell. 1985 Mar;40(3):717–724. doi: 10.1016/0092-8674(85)90220-x. [DOI] [PubMed] [Google Scholar]
- Condreay L. D., Aldrich C. E., Coates L., Mason W. S., Wu T. T. Efficient duck hepatitis B virus production by an avian liver tumor cell line. J Virol. 1990 Jul;64(7):3249–3258. doi: 10.1128/jvi.64.7.3249-3258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T., Nomura K., Hirayama Y., Kitagawa T. Establishment and characterization of a chicken hepatocellular carcinoma cell line, LMH. Cancer Res. 1987 Aug 15;47(16):4460–4464. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Li J. S., Cova L., Buckland R., Lambert V., Deléage G., Trépo C. Duck hepatitis B virus can tolerate insertion, deletion, and partial frameshift mutation in the distal pre-S region. J Virol. 1989 Nov;63(11):4965–4968. doi: 10.1128/jvi.63.11.4965-4968.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Seal G., Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980 Dec;36(3):829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. The preS1 protein of hepatitis B virus is acylated at its amino terminus with myristic acid. J Virol. 1987 May;61(5):1672–1677. doi: 10.1128/jvi.61.5.1672-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh J. C., Sninsky J. J., Summers J. W., Schaeffer E. Characterization of a pre-S polypeptide on the surfaces of infectious avian hepadnavirus particles. J Virol. 1987 May;61(5):1384–1390. doi: 10.1128/jvi.61.5.1384-1390.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh J. C., Summers J. W. Infection and uptake of duck hepatitis B virus by duck hepatocytes maintained in the presence of dimethyl sulfoxide. Virology. 1989 Oct;172(2):564–572. doi: 10.1016/0042-6822(89)90199-2. [DOI] [PubMed] [Google Scholar]
- Radziwill G., Tucker W., Schaller H. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J Virol. 1990 Feb;64(2):613–620. doi: 10.1128/jvi.64.2.613-620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Kuhn C., Guhr B., Mattaliano R. J., Schaller H. Biochemical and immunological characterization of the duck hepatitis B virus envelope proteins. J Virol. 1987 Jul;61(7):2280–2285. doi: 10.1128/jvi.61.7.2280-2285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smith P. M., Horwich A. L. Hepadnavirus envelope proteins regulate covalently closed circular DNA amplification. J Virol. 1990 Jun;64(6):2819–2824. doi: 10.1128/jvi.64.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smith P. M., Huang M. J., Yu M. S. Morphogenetic and regulatory effects of mutations in the envelope proteins of an avian hepadnavirus. J Virol. 1991 Mar;65(3):1310–1317. doi: 10.1128/jvi.65.3.1310-1317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttleman J. S., Pugh J. C., Summers J. W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986 Apr;58(1):17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka O., Omata M., Ito Y. Expression of pre-S1, pre-S2, and C proteins in duck hepatitis B virus infection. Virology. 1988 Nov;167(1):82–86. doi: 10.1016/0042-6822(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Yu M., Summers J. Multiple functions of capsid protein phosphorylation in duck hepatitis B virus replication. J Virol. 1994 Jul;68(7):4341–4348. doi: 10.1128/jvi.68.7.4341-4348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]