Abstract
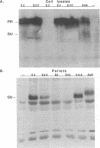
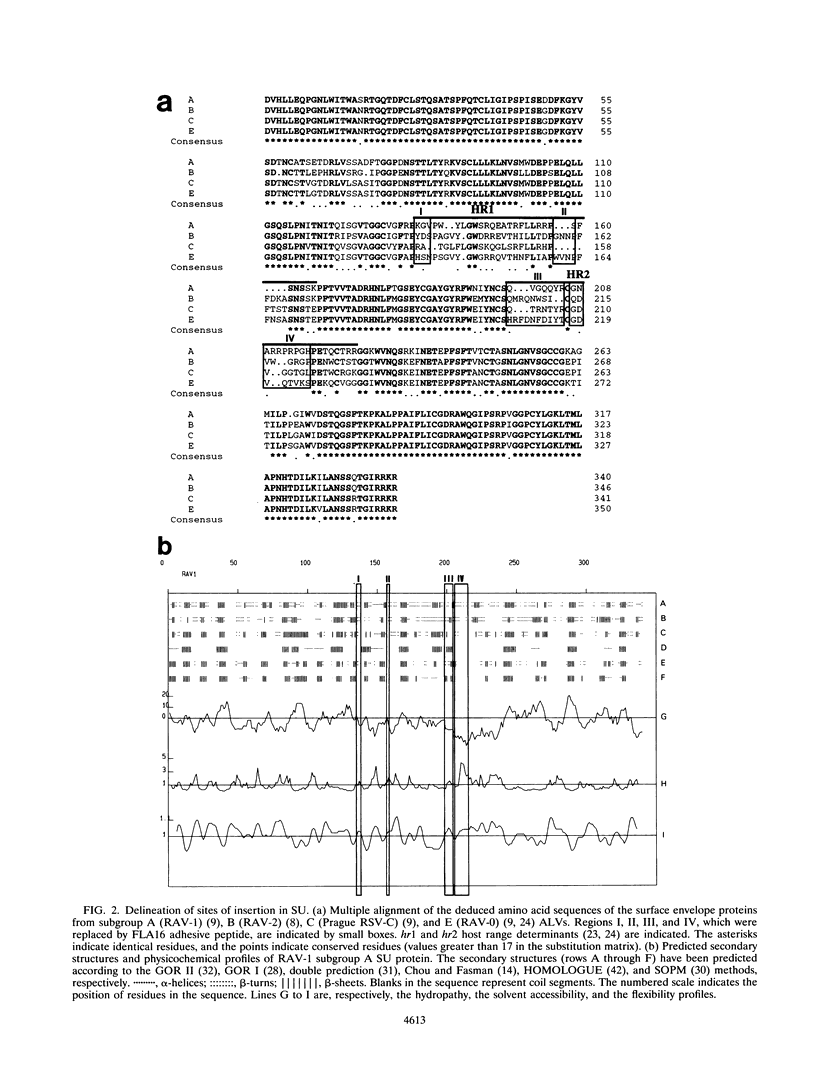
On the basis of theoretical structural and comparative studies of various avian leukosis virus SU (surface) envelope proteins, we have identified four small regions (I, II, III, and IV) in their receptor-binding domains that could potentially be involved in binding to receptors. From the envelope gene of an avian leukosis virus of subgroup A, we have constructed a set of SU mutants in which these regions were replaced by the coding sequence of FLA16, a 16-amino-acid RGD-containing peptide known to be the target for several cellular integrin receptors. Helper-free retroviral particles carrying a neo-lacZ retroviral vector were produced with the mutant envelopes. SU mutants in which regions III and IV were substituted yielded normal levels of envelope precursors but were not detectably processed or incorporated in viral particles. In contrast, substitutions in regions I and II did not affect the processing and the viral incorporation of SU mutants. When FLA16 was inserted in region II, it could be detected with antibodies against FLA16 synthetic peptide, but only when viral particles were deglycosylated. Viral particles with envelopes mutated in region I or II were able to infect avian cells through the subgroup A receptor at levels similar to those of the wild type. When viruses with envelopes containing FLA16 peptide in region II were applied to plastic dishes, they were found to promote binding of mammalian cells resistant to infection by subgroup A avian leukosis viruses but expressing the integrins recognized by FLA16. Deglycosylated helper-free viruses obtained by mild treatment with N-glycosidase F have been used to infect these mammalian cells, and infections have been monitored by neomycin selection. No neomycin-resistant clones could be obtained after infection by viruses with wild-type envelopes. Conversely, colonies were obtained after infection by viruses with envelopes bearing FLA16 in region II, and the genome of the retroviral vector was found correctly integrated in cell DNA of these colonies. By using a blocking peptide containing the minimal adhesive RGD sequence contained in FLA16, we have shown that preincubation of target cells could specifically inhibit infection by viruses with FLA16.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aumailley M., Gerl M., Sonnenberg A., Deutzmann R., Timpl R. Identification of the Arg-Gly-Asp sequence in laminin A chain as a latent cell-binding site being exposed in fragment P1. FEBS Lett. 1990 Mar 12;262(1):82–86. doi: 10.1016/0014-5793(90)80159-g. [DOI] [PubMed] [Google Scholar]
- Aumailley M., Mann K., von der Mark H., Timpl R. Cell attachment properties of collagen type VI and Arg-Gly-Asp dependent binding to its alpha 2(VI) and alpha 3(VI) chains. Exp Cell Res. 1989 Apr;181(2):463–474. doi: 10.1016/0014-4827(89)90103-1. [DOI] [PubMed] [Google Scholar]
- Aumailley M., Nurcombe V., Edgar D., Paulsson M., Timpl R. The cellular interactions of laminin fragments. Cell adhesion correlates with two fragment-specific high affinity binding sites. J Biol Chem. 1987 Aug 25;262(24):11532–11538. [PubMed] [Google Scholar]
- Bates P., Young J. A., Varmus H. E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993 Sep 24;74(6):1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- Battini J. L., Heard J. M., Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992 Mar;66(3):1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J. V., Schwarz R. T. Processing of gPr92env, the precursor to the glycoproteins of Rous sarcoma virus: use of inhibitors of oligosaccharide trimming and glycoprotein transport. Virology. 1984 Jan 15;132(1):95–109. doi: 10.1016/0042-6822(84)90094-1. [DOI] [PubMed] [Google Scholar]
- Bova-Hill C., Olsen J. C., Swanstrom R. Genetic analysis of the Rous sarcoma virus subgroup D env gene: mammal tropism correlates with temperature sensitivity of gp85. J Virol. 1991 Apr;65(4):2073–2080. doi: 10.1128/jvi.65.4.2073-2080.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova C. A., Manfredi J. P., Swanstrom R. env genes of avian retroviruses: nucleotide sequence and molecular recombinants define host range determinants. Virology. 1986 Jul 30;152(2):343–354. doi: 10.1016/0042-6822(86)90137-6. [DOI] [PubMed] [Google Scholar]
- Bova C. A., Olsen J. C., Swanstrom R. The avian retrovirus env gene family: molecular analysis of host range and antigenic variants. J Virol. 1988 Jan;62(1):75–83. doi: 10.1128/jvi.62.1.75-83.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. C., Friedmann T., Driever W., Burrascano M., Yee J. K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Different murine cell lines manifest unique patterns of interference to superinfection by murine leukemia viruses. Virology. 1985 Feb;141(1):119–129. doi: 10.1016/0042-6822(85)90188-6. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cosset F. L., Girod A., Flamant F., Drynda A., Ronfort C., Valsesia S., Molina R. M., Faure C., Nigon V. M., Verdier G. Use of helper cells with two host ranges to generate high-titer retroviral vectors. Virology. 1993 Mar;193(1):385–395. doi: 10.1006/viro.1993.1135. [DOI] [PubMed] [Google Scholar]
- Cosset F. L., Legras C., Chebloune Y., Savatier P., Thoraval P., Thomas J. L., Samarut J., Nigon V. M., Verdier G. A new avian leukosis virus-based packaging cell line that uses two separate transcomplementing helper genomes. J Virol. 1990 Mar;64(3):1070–1078. doi: 10.1128/jvi.64.3.1070-1078.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosset F. L., Legras C., Thomas J. L., Molina R. M., Chebloune Y., Faure C., Nigon V. M., Verdier G. Improvement of avian leukosis virus (ALV)-based retrovirus vectors by using different cis-acting sequences from ALVs. J Virol. 1991 Jun;65(6):3388–3394. doi: 10.1128/jvi.65.6.3388-3394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosset F. L., Ronfort C., Molina R. M., Flamant F., Drynda A., Benchaibi M., Valsesia S., Nigon V. M., Verdier G. Packaging cells for avian leukosis virus-based vectors with various host ranges. J Virol. 1992 Sep;66(9):5671–5676. doi: 10.1128/jvi.66.9.5671-5676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Dong J. Y., Dubay J. W., Perez L. G., Hunter E. Mutations within the proteolytic cleavage site of the Rous sarcoma virus glycoprotein define a requirement for dibasic residues for intracellular cleavage. J Virol. 1992 Feb;66(2):865–874. doi: 10.1128/jvi.66.2.865-874.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Hunter E. Analysis of retroviral assembly using a vaccinia/T7-polymerase complementation system. Virology. 1993 May;194(1):192–199. doi: 10.1006/viro.1993.1249. [DOI] [PubMed] [Google Scholar]
- Dong J., Roth M. G., Hunter E. A chimeric avian retrovirus containing the influenza virus hemagglutinin gene has an expanded host range. J Virol. 1992 Dec;66(12):7374–7382. doi: 10.1128/jvi.66.12.7374-7382.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Stoye J. P., Coffin J. M. Molecular basis of host range variation in avian retroviruses. J Virol. 1985 Jan;53(1):32–39. doi: 10.1128/jvi.53.1.32-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeld D., Hunter E. Oligomeric structure of a prototype retrovirus glycoprotein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8688–8692. doi: 10.1073/pnas.85.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Julan M., Roux P., Carillo S., Jeanteur P., Piechaczyk M. The efficiency of cell targeting by recombinant retroviruses depends on the nature of the receptor and the composition of the artificial cell-virus linker. J Gen Virol. 1992 Dec;73(Pt 12):3251–3255. doi: 10.1099/0022-1317-73-12-3251. [DOI] [PubMed] [Google Scholar]
- Gallagher P. J., Henneberry J. M., Sambrook J. F., Gething M. J. Glycosylation requirements for intracellular transport and function of the hemagglutinin of influenza virus. J Virol. 1992 Dec;66(12):7136–7145. doi: 10.1128/jvi.66.12.7136-7145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Geourjon C., Deléage G. Interactive and graphic coupling between multiple alignments, secondary structure predictions and motif/pattern scanning into proteins. Comput Appl Biosci. 1993 Feb;9(1):87–91. doi: 10.1093/bioinformatics/9.1.87. [DOI] [PubMed] [Google Scholar]
- Geourjon C., Deléage G., Roux B. ANTHEPROT: an interactive graphics software for analyzing protein structures from sequences. J Mol Graph. 1991 Sep;9(3):188-90, 167. doi: 10.1016/0263-7855(91)80008-n. [DOI] [PubMed] [Google Scholar]
- Geourjon C., Deléage G. SOPM: a self-optimized method for protein secondary structure prediction. Protein Eng. 1994 Feb;7(2):157–164. doi: 10.1093/protein/7.2.157. [DOI] [PubMed] [Google Scholar]
- Gibrat J. F., Garnier J., Robson B. Further developments of protein secondary structure prediction using information theory. New parameters and consideration of residue pairs. J Mol Biol. 1987 Dec 5;198(3):425–443. doi: 10.1016/0022-2836(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Goodman S. L., Aumailley M., von der Mark H. Multiple cell surface receptors for the short arms of laminin: alpha 1 beta 1 integrin and RGD-dependent proteins mediate cell attachment only to domains III in murine tumor laminin. J Cell Biol. 1991 May;113(4):931–941. doi: 10.1083/jcb.113.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Danos O. An amino-terminal fragment of the Friend murine leukemia virus envelope glycoprotein binds the ecotropic receptor. J Virol. 1991 Aug;65(8):4026–4032. doi: 10.1128/jvi.65.8.4026-4032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Hunter E., Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D. Determinants of the host range of feline leukaemia viruses. J Gen Virol. 1973 Aug;20(2):169–175. doi: 10.1099/0022-1317-20-2-169. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Levin J. M., Robson B., Garnier J. An algorithm for secondary structure determination in proteins based on sequence similarity. FEBS Lett. 1986 Sep 15;205(2):303–308. doi: 10.1016/0014-5793(86)80917-6. [DOI] [PubMed] [Google Scholar]
- Main A. L., Harvey T. S., Baron M., Boyd J., Campbell I. D. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992 Nov 13;71(4):671–678. doi: 10.1016/0092-8674(92)90600-h. [DOI] [PubMed] [Google Scholar]
- Miller A. D. Retrovirus packaging cells. Hum Gene Ther. 1990 Spring;1(1):5–14. doi: 10.1089/hum.1990.1.1-5. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Neda H., Wu C. H., Wu G. Y. Chemical modification of an ecotropic murine leukemia virus results in redirection of its target cell specificity. J Biol Chem. 1991 Aug 5;266(22):14143–14146. [PubMed] [Google Scholar]
- Noteborn M. H., de Boer G. F., Kant A., Koch G., Bos J. L., Zantema A., van der Eb A. J. Expression of avian leukaemia virus env-gp85 in Spodoptera frugiperda cells by use of a baculovirus expression vector. J Gen Virol. 1990 Nov;71(Pt 11):2641–2648. doi: 10.1099/0022-1317-71-11-2641. [DOI] [PubMed] [Google Scholar]
- Ott D., Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J Virol. 1992 Aug;66(8):4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Rein A. Interference grouping of murine leukemia viruses: a distinct receptor for the MCF-recombinant viruses in mouse cells. Virology. 1982 Jul 15;120(1):251–257. doi: 10.1016/0042-6822(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Roux P., Jeanteur P., Piechaczyk M. A versatile and potentially general approach to the targeting of specific cell types by retroviruses: application to the infection of human cells by means of major histocompatibility complex class I and class II antigens by mouse ecotropic murine leukemia virus-derived viruses. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9079–9083. doi: 10.1073/pnas.86.23.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. J., Hawkins R. E., Winter G. Retroviral vectors displaying functional antibody fragments. Nucleic Acids Res. 1993 Mar 11;21(5):1081–1085. doi: 10.1093/nar/21.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Weiss R. A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990 May;176(1):58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- White J. M. Integrins as virus receptors. Curr Biol. 1993 Sep 1;3(9):596–599. doi: 10.1016/0960-9822(93)90007-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Adhesive recognition sequences. J Biol Chem. 1991 Jul 15;266(20):12809–12812. [PubMed] [Google Scholar]
- Young J. A., Bates P., Varmus H. E. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J Virol. 1993 Apr;67(4):1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. A., Bates P., Willert K., Varmus H. E. Efficient incorporation of human CD4 protein into avian leukosis virus particles. Science. 1990 Dec 7;250(4986):1421–1423. doi: 10.1126/science.2175047. [DOI] [PubMed] [Google Scholar]
- Zanetti M., Filaci G., Lee R. H., del Guercio P., Rossi F., Bacchetta R., Stevenson F., Barnaba V., Billetta R. Expression of conformationally constrained adhesion peptide in an antibody CDR loop and inhibition of natural killer cell cytotoxic activity by an antibody antigenized with the RGD motif. EMBO J. 1993 Nov;12(11):4375–4384. doi: 10.1002/j.1460-2075.1993.tb06122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Závada J. The pseudotypic paradox. J Gen Virol. 1982 Nov;63(Pt 1):15–24. doi: 10.1099/0022-1317-63-1-15. [DOI] [PubMed] [Google Scholar]