Abstract
The α subunit (Gα) of heterotrimeric G proteins is a major determinant of signaling selectivity. The Gα structure essentially comprises a GTPase “Ras-like” domain (RasD) and a unique α-helical domain (HD). We used the vertebrate phototransduction model to test for potential functions of HD and found that the HD of the retinal transducin Gα (Gαt) and the closely related gustducin (Gαg), but not Gαi1, Gαs, or Gαq synergistically enhance guanosine 5′-γ[-thio]triphosphate bound Gαt (GαtGTPγS) activation of bovine rod cGMP phosphodiesterase (PDE). In addition, both HDt and HDg, but not HDi1, HDs, or HDq attenuate the trypsin-activated PDE. GαtGDP and HDt attenuation of trypsin-activated PDE saturate with similar affinities and to an identical 38% of initial activity. These data suggest that interaction of intact Gαt with the PDE catalytic core may be caused by the HD moiety, and they indicate an independent site(s) for the HD moiety of Gαt within the PDE catalytic core in addition to the sites for the inhibitory Pγ subunits. The HD moiety of GαtGDP is an attenuator of the activated catalytic core, whereas in the presence of activated GαtGTPγS the independently expressed HDt is a potent synergist. Rhodopsin catalysis of Gαt activation enhances the PDE activation produced by subsaturating levels of Gαt, suggesting a HD-moiety synergism from a transient conformation of Gαt. These results establish HD-selective regulations of vertebrate retinal PDE, and they provide evidence demonstrating that the HD is a modulatory domain. We suggest that the HD works in concert with the RasD, enhancing the efficiency of G protein signaling.
Heterotrimeric G proteins play a central role in many cell-signaling processes (1). These signaling proteins are members of an extensive superfamily of GTP-binding regulatory proteins characterized by a conserved GTP-binding motif (2). The G proteins differ from the monomeric members of this family in a distinct heterotrimeric quaternary structure, including a βγ subunit dimer complexed with the GTP-binding α subunit (1, 3). The α subunit (Gα) is also distinct from other GTP-binding proteins in the presence of a unique folding motif, the α-helical domain (HD), which is characteristic of G proteins (4, 5). Although the α subunit has received the most attention because most of the known structural determinants of receptor–G protein or effector–G protein interactions reside in Gα proteins (1, 3, 6), the Gβγ also is involved in receptor recognition and effector modulation (7). Solutions for the crystal structures of the α subunits of transducin (Gt) and Gi revealed that these proteins fold into two essentially separate domains, the conserved GTPase or “Ras-like” domain (RasD) and the unique α-helical domain (HD) (4, 5). To date, all sites for Gα subunit interactions with receptors and effectors have been mapped to the RasD and the α amino-terminal sequence (1, 3, 4). Little is known about the corresponding function(s) of the HD. The HD is unique to the heterotrimeric G proteins, whereas a RasD is present in all of the members of the GTPase superfamily. Comparison of the amino acid sequences reveals that diversity in the HD is remarkably greater than in the RasD among Gα families (ref. 5; Fig. 1). These observations suggest some Gα-specific function(s) for the HD moiety. Identification of the role of HD has proved elusive. The divergent sequences of HD (Fig. 1) have led to the proposal that it may serve as an effector-recognition domain (8). Various other possible functions for the HD have been postulated, including increasing the affinity of GTP binding (9), acting as a tethered intrinsic GTPase activating protein (10, 11), participating in effector recognition (5, 12, 13), participating in the inactive-active conformational transitions of Gα (14), and regulating Gα oligomerization (15, 16).
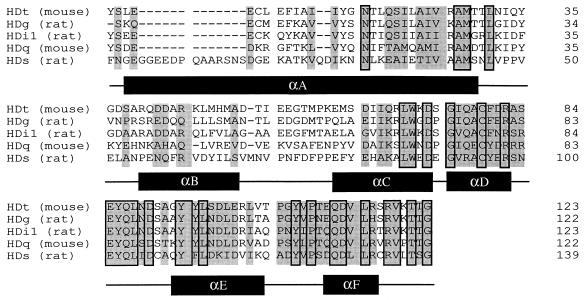
Figure 1.
Sequence alignment for Gα HDs. The amino acid sequences for the helical domains from Gαt, Gαg, Gαi1, Gαq, and Gαs are compared. The secondary structure (from αA to αF) corresponding to Gαt/αi1 (4) is shown below the aligned sequences. Conserved residues at equivalent positions are shaded. Amino acid identities are boxed.
We used the retinal signal-transduction model to investigate the potential function of the HD of transducin α subunit (Gαt). We selected this system because the molecular components of vertebrate phototransduction have been extensively characterized, as has the in vitro biochemistry for all known Gt coupled interactions (reviewed in refs. 17 and 18). The activation of the retinal rod Gαt by photoreceptor rhodopsin can be reconstituted with preparations of homogenous receptor in native disc membranes of the rod outer segment (19); Gαt regulated activation of the unique retinal cGMP phosphodiesterase (PDE) has also been examined extensively (18). The nonactivated PDE consists of a catalytic core (Pαβ) and two inhibitory Pγ subunits (20–23). The Pαβγ2 (i.e., holoPDE) remains inactive until it is activated by GTP-bound Gαt (GαtGTP). Although much has been learned about signal flow from the photoexcited receptor to the sensory synapse (24), some mechanistic details of this signaling still remain unelucidated. It has been shown that the HD of Gαs (HDs) can fold independently of the RasD (10, 12). Here we report the successful expression and purification of the HD proteins from the major Gα families. With the expressed HD proteins we establish a HD-selective interaction with the transducin effector, PDE.
MATERIALS AND METHODS
Cloning and Expression Constructs.
All of the HD constructs were generated by oligonucleotide-directed mutagenesis with influenza virus hemagglutinin (HA) tags (YPYDVDYA) at their amino termini. All constructs were confirmed by DNA sequencing, and the corresponding expressed proteins were confirmed by amino-terminal amino acid sequencing. The HDg (rat) expression plasmid was the first constructed, and its construct intermediate served as a basis for engineering the other HD constructs. Briefly, we created versatile HD expression vectors based on the gustducin construct as follows. To introduce new sites for excising HD from the corresponding cDNA sequence (25), a unique SnaBI site in the gustducin-containing cloning vector located within the region equivalent to transducin linker I (9) was created by using PCR with the primers 5′-GGGGTACCGCTGATCAACTGCCCGTCCTCTAACAG-3′ and 5′-GGAATTCGATGCATTCTTGTTTTACGTAACCATTCTTGTGGATG-3′. To introduce a stop codon into the 3′ end of the HD sequence, the HD region was amplified by using PCR with the primers 5′-CGGGATCCATGGCAAACACACTAGAAGATGGT-3′ and 5′-GCTCTAGATCAACCAG TGGTTTTCACACGGGAATGTAGAACGTCT-3′ and subcloned into pSE420 (Invitrogen). The HD constructs were tagged with HA at their amino termini by inserting the 9-aa epitope (YPYDVPDYA) into NcoI/SnaBI sites of pSE420 by using Klenow DNA polymerase-mediated 5′ → 3′ polynucleotide synthesis with the oligonucleotides 5′-CATGCCATGGGATACCCATACGACGTCCCAGACTACG-3′ and 5′-GGAATTCTACGTAAGCGTAGTCTGGGACGTCGTATGGGTATC-3′. The resultant plasmid, termed pSEHA, served as the vector for generating the other HD constructs as well. Finally, the HD coding sequence was excised with SnaBI and ligated to the SnaBI fragment of pSEHA to create HA-tagged HDg.
For each of the HDt mouse (26), HDq mouse (27), HDi1 rat (28), and HDs−L rat (29) constructs, the DNA sequence corresponding to the HD between linker I and II (9) was amplified by PCR using primers, respectively, 5′-ATTATCCACCAGGACGGGTACGTACTGGAGGAATGCCTCGAGTTC-3′ and 5′-GGAATTCTCAACCAGTGGTTTTGACACGAGAACG-3′ (HDt); 5′-CACGGGTCGGGCTACGTAGACGAAGACAAGCGCG GCTTC-3′ and 5′-GGAATTCTTACCCTGTAGTGGGGACTCGAAC-3′ (HDq); 5′-GAGGCTGGCTACGTAGAGGAAGAGTGTAAGCAG-3′ and 5′-GGAATTCTTATCCCGTGGTTTTCACTCTAGTTCTG-3′ (HDi1); and 5′-TCCCCCGGGTTTAACGGAGAGGGCGGCG-3′ and 5′-GGACTAGTTTATCCAGAGGTCAGGACGCGGCAG-3′ (HDs).
Purification of the HD Proteins.
The proteins HDg, HDi1, HDq, and HDs were expressed in strain M15 (pREP4) obtained from Qiagen (Chatsworth, CA), and HDt was produced in strain BL21(DE3); the cells were harvested according to a modified procedure from ref. 30. The isolation of all of the HD proteins was accomplished by sequential chromatography over DEAE-Sephadex A-25 (Pharmacia) and elution with a linear gradient of 50–700 mM NaCl; AcA54 (IBF Biotechnics, Columbia, MD) gel exclusion; FPLC monoQ (Pharmacia) eluted with a linear gradient of 100–500 mM NaCl, and Fast Protein Liquid Chromatography (FPLC) Superdex HR-75 (Pharmacia) size exclusion. The distribution of the HD protein was determined by immunoblotting using anti-HA monoclonal antibody 12CA5 (Boehringer Mannheim). The HD proteins eluted from DEAE and MonoQ at distinct NaCl concentrations based on their differing charges. After the final Superdex HR-75 chromatography, the HD-containing fractions were pooled, diluted to a NaCl concentration less than 7 mM with 20 mM Mops (pH 7.5) and 1 mM DTT, and concentrated in an Amicon pressure cell on YM10 membranes, then stored at −80°C or in the same solution containing 35% glycerol (vol/vol) at −20°C for short-term storage. The chemical identities of the purified HD proteins were confirmed by amino-terminal amino acid sequencing and fast atom bombardment/mass spectroscopy. The proper folding structures were verified by circular dichroism spectra. Protein concentrations were measured by using the Bradford protein assay (Bio-Rad) using BSA as standard.
Purification of PDE, Preparation of tPDE and Pγ Subunits, and PDE Assay.
PDE was isolated from the bovine rod outer segment (20). To prepare trypsin-activated PDE (tPDE), 27 μl of 10 μM purified PDE was incubated with 120 μl of agarose-immobilized N-tosyl-l-phenylalanine chloromethyl ketone (TPCK)–trypsin (Pierce) in a solution containing 50 mM Mops (pH 7.5), 5 mM NaCl, and 2 mM DTT for 17 min at 30°C. The reaction was terminated by centrifugation at 12,000 × g for 5 min at 4°C to precipitate the agarose beads. When this method was used, more than 97% of the initial GαtGTPγS-activated activity was obtained, and GαtGTPγS no longer activated the PDE. Pγ subunits were prepared from purified PDE according to the procedure of Hurley and Stryer (21). PDE activity was quantified as inorganic phosphate released by 5′-nucleotidase digestion of GMP as measured by molybdate-dependent absorbence at 790 nm. The assay followed the modified procedure from ref. 31. Phosphate production was found to be linear with time and with PDE concentration under all conditions reported in this study and up to 87 nmol of cGMP hydrolyzed.
Other Methods.
The GαtGDP was isolated from GTP-eluted transducin by chromatography on Blue Sepharose CL-6B (Pharmacia) as modified from Yamazaki et al. (32). The GαtGTPγS was prepared from the purified GαtGDP as described (19). Immunoblotting for HA-tagged HD was done with monoclonal antibody 12CA5 (Boehringer Mannheim) and enhanced chemiluminescence detection (Amersham).
Materials.
Immobilized trypsin (beaded agarose) and N-hydroxysulfosuccinimide were purchased from Pierce. cGMP, GTPγS, and 5′-nucleotidase were from Sigma.
RESULTS
Design and Characterization of the Purified HD Proteins.
We designed HDs based on the crystallographic data of Gαt/Gαi1 chimera complexed with GTPγS⋅Mg2+ and with GDP (4, 9). The Gα crystal structures show three distinct structural components: a HD consisting of a long central helix (αA) surrounded by 5 shorter helices (αB–αF) (4); a RasD consisting of a six-stranded β-sheet surrounded by six helices (α1–α5) and αG; and an amino-terminal segment. Connection of the HD to the other two components is achieved by linker 1 and linker 2 polypeptides. Therefore, we engineered HDs in such a way that they consisted of αA–αF with their amino termini capped by the linker 1 polypeptides and carboxy termini by the linker 2 polypeptides†. The amino acid sequences of the HD proteins used in these investigations are shown in Fig. 1. Consistent with previous reports of the HD being an independently folded domain (10, 12), the HDs obtained from Gαs, Gαq, Gαi1, Gαt, and Gαg were expressed, although with different yields, as stable and soluble proteins with predominantly α-helical secondary structures confirmed by circular dichroism spectral analyses (unpublished data).
Selective HD Interactions with PDE.
Our basic approach to identify potential interaction(s) of the HD proteins was to test for the competitive interference in the cascade of rhodopsin-catalyzed activation of the PDE. We predicted that the receptor or effector contacts with the HD moiety of Gα (if any) would be insufficient to elicit function independent of the RasD. Therefore, HD should competitively inhibit the interactions of intact Gα with these signaling components. Our initial investigation of rhodopsin–Gαt interaction found no evidence for HDt binding to rhodopsin or Gβγ (data not shown). Therefore, we set out to investigate the potential involvement of HD in Gα–effector interaction.
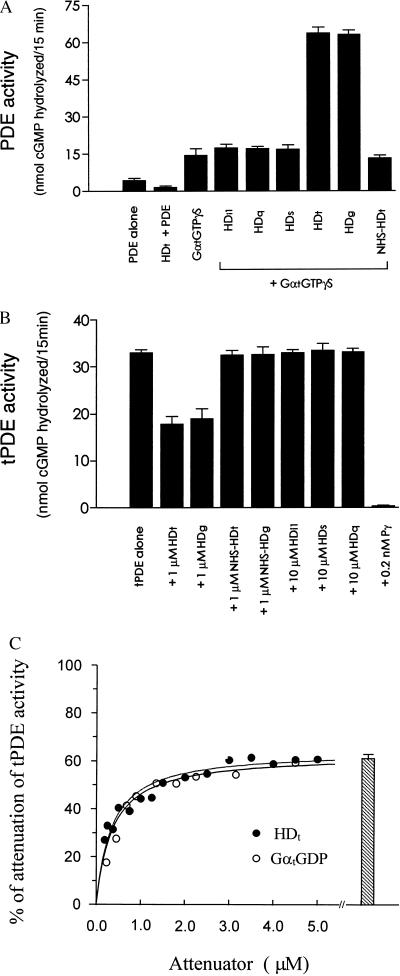
The rod cGMP-PDE can be stimulated in vitro by the activator GαtGTPγS (33). When we examined the HD reactivity toward the PDE, to our surprise, we found that rather than competing with GαtGTPγS, the HDt caused an enhanced PDE activation (Fig. 2A). HDi1 (Gαi family), which is closely related to HDt; HDq, or HDs elicited no enhanced activation under the same conditions (Fig. 2A), indicating a selective interaction between HDt and the PDE enzyme. In addition, the HDg (the HD of Gαg) stimulated to the same extent as the HDt (Fig. 2A). To exclude the possibility that the HD effect on augmentation of the enzyme activation may be attributed to denatured or unfolded proteins, we tested chemically modified HDt. Modification of the HDt with N-hydroxysuccinimide (NHS) abrogated all enhancement of the PDE activation (Fig. 2 A and B). This result indicates that the HDt–PDE interaction is conformation-dependent.
Figure 2.
Selective HD regulation of PDE. (A) Selectivity of HD synergy with GαtGTPγS. The enzymatic activity of PDE (0.5 nM) was determined with the indicated additions to the reactions. The concentrations for HDi1, HDq, HDs, and NHS-HDt were 2.0 μM, HDt and HDg were 1.0 μM, and GαtGTPγS was 0.45 μM. Error bars indicate ±SEM derived from values obtained in three independent experiments, with each determination performed in triplicate. PDE activity assay was performed as described in Materials and Methods. The GαtGTPγS was prepared from the purified GαtGDP as described (47). (B) Selective interaction of the HDs with PDE catalytic core. Reactivity of the HD proteins isolated from different Gα families toward PDE catalytic core was determined with 0.2 nM tPDE in the presence of the indicated HD concentrations. Limited trypsinization to produce tPDE was performed as described in Materials and Methods. Each value is the mean ± SEM of data obtained from three independent experiments. (C) Attenuation of the catalytic core enzyme by HDt and GαtGDP. Attenuation of 0.2 nM tPDE was determined in the presence of the indicated concentrations of either HDt (•) or GαtGDP (○). The activity for each condition is expressed as the fractional inhibition of the activity assayed for the tPDE alone (34.2 ± 1 nmol of cGMP hydrolyzed). The bar presents the fractional inhibition obtained in the presence of 4.5 μM GαtGDP and 5 μM HDt. The error bars are ±SEM for values obtained in three independent experiments. The curves are representative of the saturations from three independent experiments.
Furthermore, both the HDt and the HDg directly interact with the PDE catalytic subunits (Pαβ), as demonstrated by their attenuation of tPDE (Fig. 2B). An identical pattern of selectivity was obtained for the HDt attenuation of Pαβ as found for the synergistic activation; the HDs obtained from the members of the other G protein families showed no attenuation of Pαβ activity (Fig. 2B). Our data for HD attenuation of Pαβ are similar to those obtained previously for GαtGDP attenuation of the enzyme (34). In Fig. 2C, we compare GαtGDP and HDt attenuation of tPDE. At saturating concentrations, both GαtGDP and HDt only partially inhibit tPDE (62 ± 4%, mean ± SD, n = 3 for both GαtGDP and HDt) eliminating a competitive mechanism for this attenuation. The apparent affinities obtained in this experiment of 500 nM for GαtGDP and 330 nM for HDt attenuation are nearly equal. Furthermore, the coaddition of saturating concentrations of GαtGDP and HDt produced no additional inhibition over that found for each independently (Fig. 2C). These data suggest that the attenuation of Pαβ by GαtGDP may be conferred by its HD moiety.
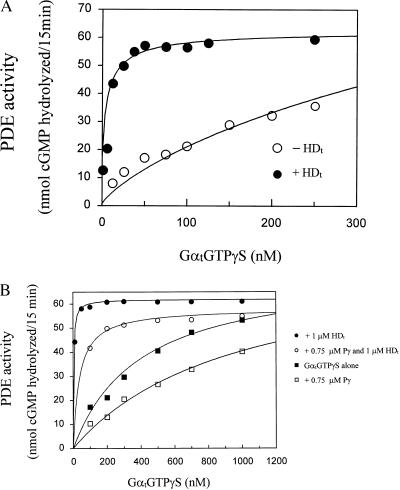
To gain insight into the molecular basis of the synergy conferred by the HDt, we examined the HDt influence on the saturation of PDE activation by GαtGTPγS. As shown in Fig. 3A, the HDt remarkably increases the apparent affinity of PDE for GαtGTPγS, decreasing the apparent Kd values from 460 nM to 3 nM. In separate experiments using higher concentrations of GαtGTPγS (up to 3 μM) to reach saturation, we determined the apparent Kd for PDE activation to be 500 ± 70 nM (mean ± SD, n = 5), and the Kd in the presence of HDt to be 2.5 ± 1.2 nM (n = 5). This >150-fold enhancement of affinity suggests an allosteric regulation of PDE by the HDt. These results, along with the data showing a direct interaction of the HDt with Pαβ (Fig. 2 B and C) strongly argue the existence of at least two distinct sites in PDE for binding of Gαt—a HD site on the Pαβ as well as the previously defined binding sites for Pγ. We suggest that the HD moiety of Gαt may function as an allosteric modulator of the Pγ interaction with the Gαt RasD. The principal mechanism described for PDE activation involves the Gαt-mediated release of inhibition imposed by two inhibitory Pγ subunits on the Pαβ catalytic core (17, 35). This Gαt–Pγ interaction has been attributed to binding of the RasD in the active GTP-bound conformation to Pγ (4, 36, 37). The HDt synergy with the GαtGTPγS is not prevented by Pγ. Fig. 3B shows that 1 μM HDt enhances the affinity for GαtGTPγS activation of PDE even in the presence of 750 pM Pγ, a concentration more than 30-fold greater than the Kd of Pγ for Pαβ. These data argue that the HD acts as a modulator rather than a competitor for the Pγ–Pαβ interaction.
Figure 3.
Enhancement of the GαtGTPγS-elicited PDE activation by HDt. (A) HDt enhancement of the GαtGTPγS affinity for the PDE. The activity of PDE (0.5 nM) was determined in the presence of the indicated concentrations of GαtGTPγS with (•) or without (○) addition of 1 μM HDt. The data are representative of three independent experiments. The basal activity of the PDE (4.7 nmol of cGMP) was subtracted for calculation of the curve fits. Curve fitting was performed with nonlinear least squares criteria by using Graphpad prism software. (B) Independence of the HDt and Pγ regulation of PDE. The activity of PDE (0.5 nM) was determined with the indicated concentrations of GαtGTPγS either alone (■) or in the presence of 750 pM Pγ (□), 1.0 μM HDt (•), or 750 pM Pγ with 1.0 μM HDt (○). The data are representative of three independent experiments. The inhibitory Pγ subunit was prepared from the purified PDE according to the procedure described by Hurley and Stryer (21). The basal activity of the PDE (3.4 nmol of hydrolyzed cGMP) was subtracted from all values.
The HD Moiety Function in Intact α Subunit.
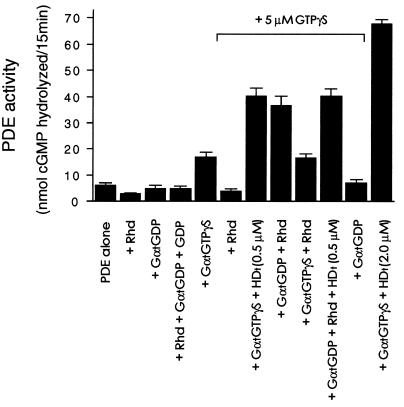
A correlation between our data from the independently expressed HDt and its function within the intact Gαt subunit has been provided by examination of the interaction of the HDt and GαtGDP with the PDE catalytic subunit (Pαβ) (Fig. 2 B and C). This kinetic similarity between HDt and GαtGDP strongly argues that the GαtGDP reactivity toward the core enzyme is a result of its HD moiety. However, unlike HDt protein, GαtGDP is unable to enhance the PDE activity under the same conditions for HDt synergy with GαtGTPγS. This suggests a conformational dependence of the HD moiety for synergy of the PDE activation by the RasD moiety of GαtGTPγS. This hypothesis is difficult to assess because the RasD of GαtGTPγS is in its activated conformation for binding to Pγ. We have estimated the half-saturating concentration of the isolated HDt at 300–500 nM, which is near the half-saturation of GαtGTPγS activation in the absence of HDt. The contribution of the HDt moiety as a synergist of PDE activation is difficult to segregate from the RasD interaction with Pγ subunit at these concentrations. To examine the possibility of conformational dependence of the HD moiety of intact Gαt subunits, we designed experiments using rhodopsin to catalyze the conformational transitions of Gαt. The experiment in Fig. 4 compares the PDE activation produced by 125 nM GαtGTPγS to that produced by 125 nM GαtGDP in the presence of rhodopsin and GTPγS. If the only product of the rhodopsin-catalyzed reaction regulating PDE activity was GαtGTPγS, the activity produced by rhodopsin with 125 nM GαtGDP and GTPγS must be less than or equal to that of the preformed 125 nM GαtGTPγS activity. As shown in Fig. 4, in the presence of rhodopsin and GTPγS to catalyze the Gαt activation, 125 nM GαtGDP is a more potent activator of PDE than the same concentration of GαtGTPγS. The rhodopsin alone does not enhance the GαtGTPγS-mediated activation, nor does GαtGDP alone, but HDt does, demonstrating that its synergy is independent of rhodopsin catalysis. We found no activation of PDE by GαtGDP alone or in the presence of GDP and rhodopsin. This result indicates that the formation of the activated GαtGTPγS, as expected, is required for the activation of PDE. The extent of activation in the condition of rhodopsin-catalyzed activation of 125 nM GαtGDP is nearly identical to the activation by 125 nM GαtGTPγS with HDt. The fact that these activities are not limited by the capacity of the PDE is shown by the addition of 2 μM HDt with 125 nM GαtGTPγS. Together, these data suggest that the process of rhodopsin-catalyzed GDP/GTP exchange produces a synergized activation of PDE by intact Gαt subunits.
Figure 4.
Influence of rhodopsin on PDE activation by Gαt and HDt. The enzymatic activity of 0.2 nM PDE was determined with the indicated additions to the reactions. When present, rhodopsin was added at 1 μM, GαtGDP and GαtGTPγS were 125 nM, and HDt was 0.5 μM. For nucleotide exchange, 5 μM GTPγS or 5 μM GDP were added with rhodopsin as indicated. The PDE reactions were conducted for 15 min. Error bars indicate ±SEM for values obtained in three independent experiments. The GαtGDP was purified as described (32), and its concentration was determined by rhodopsin-catalyzed GDP/[35S]GTPγS exchange according to the modified method (19, 47).
DISCUSSION
In this work we have confirmed the earlier report that the HD portion of the Gαs could be expressed as a properly folded structure autonomous of the amino terminus and Ras-domain sequences of the intact protein structure (10, 12). Although the former report suggested that the HD may serve as an internal GTPase-activating protein for the Gα-Ras domain, we have identified an effector regulation by the isolated HD protein. Our data demonstrate two HD-selective interactions with PDE by using the isolated proteins in solution. HDt synergistically activates the holoPDE enzyme in conjunction with activated Gtα, and HDt attenuates the activity of the tryptically activated catalytic core enzyme. Both regulatory effects are selective for HDt and HDg; neither effect is elicited by the HDs from αs, αq, or the closely related αi1. These data reiterate the well established selectivity in the G protein regulation of this enzyme. The similarity between HDt and HDg agrees with the previous report that Gαg is functionally indistinguishable from Gαt in biochemical assays (38). It has been noted that among all of the known G protein α subunits, the Gαg shares highest amino acid identity to rod and cone Gαt subunits (39). This selectivity compellingly argues that the HD regulation is biologically appropriate rather than an artifact of the recombinant expression of these sequences.
The attenuation of tPDE activity by HDt is, in essence, identical to the inhibition of tPDE by GαtGDP, which has been reported (34). Neither HDt nor GαtGDP inhibits the tPDE activity completely. In our experiments, these both saturated at 62% inhibition, whereas the prior reports found 60–70% inhibition. These data, showing partial inhibition of the tPDE activity, exclude the possibility that the HDt and GαtGDP inhibit by competing with cGMP. Rather, these data suggest an allosteric site(s) on the catalytic core enzyme noncompetitively regulating PDE activity. Furthermore, we found nearly identical apparent affinities for both HDt and GαtGDP attenuation of the PDE catalytic core, and the two inhibitors were not additive. At saturation, the coaddition of both HDt and GαtGDP attenuated tPDE activity to the same extent as either alone. Together, these data suggest that the attenuation of tPDE by GαtGDP may be conferred by an interaction of its HD moiety. The attenuation of tPDE by GαtGDP is conformationally specific. GαtGTPγS does not inhibit tPDE (34). Rather, this conformation of Gαt is a competent form for the activation of holoPDE. The influence of HDt on the interaction of holoPDE with the activated conformation of Gαt is a profound enhancement of apparent affinity—over 150-fold. Because the extent of activation is identical by GαtGTPγS either alone or in the presence of HDt, we interpret this as an allosteric regulation of the PDE by HDt. The activation of PDE via binding of the inhibitory Pγ to the RasD moiety of GαtGTPγS has been well established (36, 37). Our data suggest that the efficiency of this process is improved by the allosteric interaction of HDt with Pαβ. Because the HDt also functions as an inhibitor of the catalytic core of PDE, and this reproduces identically the attenuation by GαtGDP, we suggest that distinct conformations of the HD moiety in Gαt may be involved in the two functions. Indeed, it has been noted that the so-called “switch IV” region within the HD undergoes conformational transition between GDP and GTPγS states of Gα (15). Our examination of the conformational dependence of HDt synergy suggests an additional conformation(s) produced during the rhodopsin-catalyzed transitions of Gαt as the basis for the synergistic activation. These data may reconcile the seemingly low affinity we report, as found by others (40–42) for the PDE activation by GαtGTP in solution experiments, as opposed to the affinities obtained in the presence of rod outer segment discs (43). Rhodopsin dynamically catalyzes the formation not only of activated GαtGTP but also of all of the intermediate states of the Gαt, including those in which the HD moiety may attain distinct conformation(s). We offer the possibility that a transient intermediate, such as the “empty state” of Gαt, provides a synergistic HD moiety, enhancing the activation by the RasD moiety of the GαtGTP, and that this conformation is more readily attained in the isolated HDt protein that we have tested. In this way, the two separate folding domains may cooperate in the overall efficiency of effector regulation, the RasD being the activator and the HD a modulator of the RasD affinity for effector interaction. Furthermore, the conformational switch of the Gαt on GTP hydrolysis produces a HD that acts as an attenuator of the activated PDE.
Our data clearly establish the interaction of the isolated HD protein with the PDE, and suggest a synergism of the two folding domains in the regulation of the downstream effector. These data provide evidence for a function of the HD of a G protein, i.e., modulation of the effector regulation by the activated Ras domain. Because the overall folding motif is essentially identical for the Gαt and Gαi proteins from the solutions of their three-dimensional structures in crystals, and all Gα proteins contain the HD, it is tempting to speculate that a similar function has been conserved for each of the proteins. The recent determinations of the three-dimensional structures for the soluble catalytic domains of adenylyl cyclases exhibit intrachain dimerization of a catalytic fold (44, 45). We have obtained hydrodynamic evidence that the retinal PL-Cβ homologue from squid retina (46) is a dimer of the 140-kDa catalytic chains (J.K.N., unpublished data). A common allosteric regulation similar to the retinal PDE may well apply to these major target enzymes for G protein signaling. We would expect that the HD modulation mechanism would represent a common signaling feature among the members of G protein families. Now that we know that a selectivity is encoded in the Gα-HD, we next want to know how it cooperates intramolecularly with Gα-RasD and intermolecularly with Gβγ and other factors to regulate G protein signaling.
Acknowledgments
We thank Dr. Gerson H. Cohen for his help and access to his protein database terminal; Dr. Howard Jaffe (National Institute of Neurological Disorders and Stroke) for performing amino acid sequencing; and Dr. Pushkar Sharma for operation of circular dichroism spectrometer. We thank Loren Chen for his able technical assistance and Drs. William Clark, Xiaoying Jian, Paul Randazzo, and Nicholas Ryba for numerous helpful discussions of this work.
ABBREVIATIONS
- HA
hemagglutinin
- HD
α-helical domain of Gα subunits
- RasD
Ras-like GTPase domain of Gα subunits
- Gαt
transducin α subunit
- Gαg
gustducin α subunit
- Gαi1
the α subunit of the adenylyl cyclase inhibiting G protein Gil
- Gαs
the α subunit of the adenylyl cyclase stimulating G protein Gs
- Gαq
the α subunit of the phospholipase Cβ stimulating G protein Gq
- PDE
bovine retinal cGMP phosphodiesterase
- tPDE
tryptically activated PDE
- NHS
N-hydroxysuccinimide
Note Added in Proof
The mechanistic implications of this work are the subject of an additional report (48).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
The linker 1 and linker 2 sequences of the designed helical domains are the first two amino acid residues (except for the HD of Gαg that possesses only one residue) at the amino terminus of αA helix, and the last seven residues at the carboxyl terminus of αF helix.
We have noted that Gαt, which was isolated by aluminum fluoride activation of transducin (47), although displaying a “basal” activity state as assessed by inability to activate holoPDE, nevertheless fails to reproduce GαtGDP attenuation of tPDE.
References
- 1. Hamm H E. Annu Rev Biochem. 1998;66:669–672. [Google Scholar]
- 2.Bourne H R, Sanders D A, McCormick F. Nature (London) 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 3.Sprang S R. Annu Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 4.Lambright D G, Noel J P, Hamm H E, Sigler P B. Nature (London) 1994;369:621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- 5.Coleman D E, Berghuis A M, Lee E, Linder M E, Gilman A G, Sprang S R. Science. 1994;265:1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- 6.Conklin B R, Bourne H R. Cell. 1993;73:631–641. doi: 10.1016/0092-8674(93)90245-l. [DOI] [PubMed] [Google Scholar]
- 7.Clapham D E, Neer E J. Annu Rev Pharmacol Toxicol. 1997;20:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 8.Masters S B, Stroud R M, Bourne H R. Protein Eng. 1986;1:47–54. [PubMed] [Google Scholar]
- 9.Noel J P, Hamm H E, Sigler P B. Nature (London) 1993;366:654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- 10.Markby D W, Onrust R, Bourne H R. Science. 1993;262:1895–1901. doi: 10.1126/science.8266082. [DOI] [PubMed] [Google Scholar]
- 11.Landis C A, Masters S B, Spada A, Pace A M, Bourne H R, Vallar L. Nature (London) 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin D R, Markby D W, Bourne H R, Kuntz I D. J Mol Biol. 1995;254:681–691. doi: 10.1006/jmbi.1995.0647. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli M, Birnbaumer L, Allende J E, Olate J. FEBS Lett. 1994;340:249–254. doi: 10.1016/0014-5793(94)80148-7. [DOI] [PubMed] [Google Scholar]
- 14.Codina J, Birnbaumer L. J Biol Chem. 1994;269:29339–29342. [PubMed] [Google Scholar]
- 15.Mixon M B, Lee E, Coleman D E, Berghuis A M, Gilman A G, Sprang S R. Science. 1995;270:954–960. doi: 10.1126/science.270.5238.954. [DOI] [PubMed] [Google Scholar]
- 16.Rodbell M. Top Cell Regul. 1992;32:1–47. doi: 10.1016/b978-0-12-152832-4.50003-3. [DOI] [PubMed] [Google Scholar]
- 17.Yarfitz S, Hurley J B. J Biol Chem. 1994;269:14329–14332. [PubMed] [Google Scholar]
- 18.Stryer L. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- 19.Fawzi A B, Northup J K. Biochemistry. 1990;29:3804–3812. doi: 10.1021/bi00467a030. [DOI] [PubMed] [Google Scholar]
- 20.Baehr W, Devlin M J, Applebury M L. J Biol Chem. 1979;254:11669–11677. [PubMed] [Google Scholar]
- 21.Hurley J B, Stryer L. J Biol Chem. 1982;257:11094–11099. [PubMed] [Google Scholar]
- 22.Deterre P, Bigay J, Forquet F, Robert M, Chabre M. Proc Natl Acad Sci USA. 1988;85:2424–2428. doi: 10.1073/pnas.85.8.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chabre M, Deterre P. Eur J Biochem. 1989;179:255–266. doi: 10.1111/j.1432-1033.1989.tb14549.x. [DOI] [PubMed] [Google Scholar]
- 24.Stryer L. J Biol Chem. 1991;266:10711–10714. [PubMed] [Google Scholar]
- 25.McLaughlin S K, Mckinnon P J, Margolskee R F. Nature (London) 1992;357:563–569. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 26.Raport C J, Dere B, Hurley J B. J Biol Chem. 1989;264:7122–7128. [PubMed] [Google Scholar]
- 27.Strathmann M, Simon M I. Proc Natl Acad Sci USA. 1990;87:9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones D T, Reed R R. J Biol Chem. 1987;262:14241–14249. [PubMed] [Google Scholar]
- 29.Itoh H, Kozasa T, Nagata S, Nakamura S, Katada T, Ui M, Iwai S, Ohtsuka E, Kawasaki H, Suzuki K, et al. Proc Natl Acad Sci USA. 1986;83:3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee E, Linder M E, Gilman A G. Methods Enzymol. 1994;237:146–164. doi: 10.1016/s0076-6879(94)37059-1. [DOI] [PubMed] [Google Scholar]
- 31.Gillespie P G, Beavo J A. Mol Pharmacol. 1989;36:773–781. [PubMed] [Google Scholar]
- 32.Yamazaki A, Hayashi F, Tatsumi M, Bitensky M W, George J S. J Biol Chem. 1987;265:11539–11548. [PubMed] [Google Scholar]
- 33.Wensel T G, Stryer L. Proteins Struct Funct Genet. 1986;1:90–99. doi: 10.1002/prot.340010114. [DOI] [PubMed] [Google Scholar]
- 34.Kroll S, Phillips W J, Cerione R A. J Biol Chem. 1989;264:4490–4497. [PubMed] [Google Scholar]
- 35.Fung B K-K, Uoung J H, Yamane H K, Griswold-Prenner I. Biochemistry. 1990;29:2657–2664. doi: 10.1021/bi00463a006. [DOI] [PubMed] [Google Scholar]
- 36.Skiba N P, Bae H, Hamm H E. J Biol Chem. 1996;271:413–424. doi: 10.1074/jbc.271.1.413. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Arshavsky V Y, Ruoho A E. J Biol Chem. 1996;271:26900–26907. doi: 10.1074/jbc.271.43.26900. [DOI] [PubMed] [Google Scholar]
- 38.Hoon M A, Northup J K, Margolskee R F, Ryba N J P. Biochem J. 1995;309:629–636. doi: 10.1042/bj3090629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Margolskee R F. BioEssays. 1993;15:645–650. doi: 10.1002/bies.950151003. [DOI] [PubMed] [Google Scholar]
- 40.Malinski J A, Wenesl T G. Biochemistry. 1992;31:9502–9512. doi: 10.1021/bi00154a024. [DOI] [PubMed] [Google Scholar]
- 41.Artemyev N O, Rarick H M, Mills J S, Skiba N P, Hamm H E. J Biol Chem. 1992;267:25067–25072. [PubMed] [Google Scholar]
- 42.Bennett N, Clerc A. Biochemistry. 1989;28:7418–7424. doi: 10.1021/bi00444a040. [DOI] [PubMed] [Google Scholar]
- 43.Fung B K-K, Stryer L. Proc Natl Acad Sci USA. 1980;77:2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tesmer J J G, Sunahara R K, Gilman A G, Sprang S R. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 45.Zhang G, Liu Y, Ruoho A E, Hurley J H. Nature (London) 1997;386:247–253. doi: 10.1038/386247a0. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell J, Gutierrez J, Northup J K. J Biol Chem. 1995;270:854–859. doi: 10.1074/jbc.270.2.854. [DOI] [PubMed] [Google Scholar]
- 47.Fawzi A, Fay D S, Murphy E A, Tamir H, Erdos J J, Northup J K. J Biol Chem. 1991;266:12194–12200. [PubMed] [Google Scholar]
- 48.Liu, W., Clark, W. A., Sharma, P. & Northrup, J. K. (1998) J. Biol. Chem., in press. [DOI] [PubMed]