Abstract
RNA polymerase I (pol I) is a nuclear enzyme whose function is to transcribe the duplicated genes encoding the precursor of the three largest ribosomal RNAs. We report a cell-free system from broccoli (Brassica oleracea) inflorescence that supports promoter-dependent RNA pol I transcription in vitro. The transcription system was purified extensively by DEAE-Sepharose, Biorex 70, Sephacryl S300, and Mono Q chromatography. Activities required for pre-rRNA transcription copurified with the polymerase on all four columns, suggesting their association as a complex. Purified fractions programmed transcription initiation from the in vivo start site and utilized the same core promoter sequences required in vivo. The complex was not dissociated in 800 mM KCl and had a molecular mass of nearly 2 MDa based on gel filtration chromatography. The most highly purified fractions contain ≈30 polypeptides, two of which were identified immunologically as RNA polymerase subunits. These data suggest that the occurrence of a holoenzyme complex is probably not unique to the pol II system but may be a general feature of eukaryotic nuclear polymerases.
Keywords: nucleolus, rDNA, gene expression
Eukaryotic nuclear RNA polymerases interact with transcription factors to recognize gene promoters and initiate transcription. Many of the required factors can be purified individually and added back together to reconstitute transcription in vitro, allowing their identification and molecular characterization (1–7). However, proteins not required under a given set of assay conditions can be overlooked, though they may play important roles in vivo. The latter insight has come, in part, from the characterization of RNA pol II holoenzyme complexes in yeast and mammals (8–12). These complexes are several megadaltons in size and include pol II core enzyme subunits, most of the general transcription factors, and a growing list of additional activities including protein kinases (13), chromatin remodeling activities(14), and proteins involved in DNA repair (12).
There has been at least one report of a pol I-containing fraction from rat cell-free extracts sufficient for accurate transcription in vitro (15), and several studies have shown that pol I transcription factors interact. For instance, the vertebrate pol I transcription factors UBF and SL1/TIF-IB interact both in solution and on the DNA (16–18). Likewise, UBF and pol I cosediment in glycerol gradients and interact on Far-Western blots (19). Two additional factors, TIF-IA and TIF-IC copurify with pol I on multiple columns (20, 21). Collectively, these results suggest that the pol I transcription factors might be associated with one another, possibly within a complex analogous to the pol II holoenzyme.
Encouraged by brief reports that cell-free rRNA gene transcription could be achieved in plant extracts (22, 23), we set out to develop a cell-free system from broccoli (Brassica oleracea) to further our studies of rRNA gene regulation in Brassica and Arabidopsis (24–28). We show that a pol I-containing activity sufficient for promoter-dependent transcription can be purified extensively, with biochemical properties suggesting a single complex. Approximately 30 polypeptides are present in fractions purified to near-homogeneity, consistent with the estimated mass of the putative holoenzyme complex and the expected complexity of the pol I transcription system.
MATERIALS AND METHODS
Preparation of Broccoli Nuclear Extracts.
Nuclear extracts were prepared from chilled (4°C) broccoli inflorescence purchased at a local supermarket, with all steps performed at 4°C. After removing stalks and large stems, each 100–125 g of inflorescence was added to 200 ml of ice-cold homogenization buffer (29) [0.44 M sucrose/1.25% Ficoll/2.5% Dextran/20 mM Hepes, pH 7.4/10 mM MgCl2/0.5% Triton X-100 supplemented just prior to use with 0.5 mM DTT/5.0 μg/ml antipain/3.5 μg/ml bestatin/5.0 μg/ml leupeptin/4.0 μg/ml pepstatin A/0.5 mM phenylmethylsulfonyl fluoride (PMSF)] and homogenized at maximum speed in a Waring blender using five pulses of 20 sec each. The homogenate was filtered through two layers of Miracloth (Calbiochem) and nuclei were pelleted at 9,400 × g for 30 min. The pellet was resuspended gently in 50 ml of resuspension buffer (RB; 50 mM Hepes, pH 7.9/20% glycerol/10 mM EGTA/10 mM MgSO4.7H2O/0.5 mM PMSF/0.5 mM DTT), and then solid NaCl was added to a final concentration of 1.0 M. The sample was mixed on a stirring motor for ≈10 min, then 1.0 ml of 50% (wt/vol) polyethylene glycol (PEG 8000) was added, and stirring continued 20 min. Following centrifugation at 17,000 × g for 30 min., the supernatant was diluted 8-fold with RB buffer. Proteins were precipitated with solid (NH4)2SO4 added to 0.33 g/ml over a 20-min period, followed by incubation at 4°C for 1 hr and centrifugation at 17,000 × g for 30 min. Precipitated proteins were resuspended in RB containing 100 mM KCl (RB100) to a final volume of 12 ml per 100–125 g of starting tissue. Typical protein concentrations were ≈1.5–2.0 mg/ml as estimated by the method of Bradford (30) using BSA as the standard.
Assay for Nonspecific RNA Polymerase Activity.
Promoter-independent (nonspecific) polymerase activity was measured using standard assays (31–33). Crude extract or column fraction (20 μl) was mixed with 20 μl of 2× reaction mix containing 100 mM Hepes-KOH (pH 7.9), 100 mM KCl, 4 mM MnCl2, 1 mM each rATP, UTP, rCTP, 0.08 mM unlabeled rGTP, 2 mg/ml BSA, 50 μg/ml sheared calf thymus DNA, 100 μg/ml α-amanitin (Sigma), and 10 μCi (1 Ci = 37 GBq) α-labeled [32P]rGTP ([32P]UTP was used initially with similar results). After 30 min at 30°C, reactions were spotted on DEAE (Whatman DE81) filters. Unincorporated label was removed with four washes in 0.5 M sodium phosphate (5 min each) followed by washes in 95% ethanol (twice) and diethyl ether to aid drying. Incorporated radioactivity was determined by scintillation counting. Background was determined from mock reactions lacking added protein.
Purification of RNA Pol I.
Nuclear extracts were purified using three or four chromatography steps (see Figs. 1A and 4A). The first two steps were the same in both schemes. For the data of Figs. 1, 2, 3, ≈50 ml of ammonium sulfate-concentrated nuclear extract was loaded onto a 20 ml DEAE Sepharose CL-6B (Pharmacia) column equilibrated in RB100. The flow-through was collected and the column washed with RB100. Bound proteins were eluted with successive RB200 and RB400 steps. The 400 mM KCl-eluted fraction (DEAE 400) was dialyzed against RB100 then loaded onto a 2 ml Biorex 70 (Bio-Rad) column equilibrated in RB100. The column was washed with RB100 and eluted with RB800. This Biorex 800 fraction was dialyzed against column buffer (CB; 25 mM Hepes, pH 7.9/20% glycerol/0.1 mM EDTA/0.5 mM DTT) containing 100 mM KCl (CB100) and then subjected to chromatography on a 2 ml analytical Mono Q fast protein liquid chromatography (FPLC) column (Pharmacia). The Mono Q column was washed with CB100 then eluted with a 20 ml linear gradient from CB100 to CB600. Fractions (1 ml) were dialyzed against RB100 then assayed for nonspecific and promoter-dependent transcription.
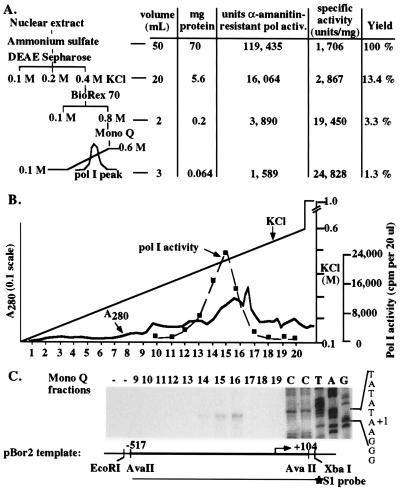
Figure 1.
Purification of broccoli (B. oleracea) fractions that program accurate transcription initiation in vitro. (A) Purification scheme and summary data. Approximately 70 mg of nuclear protein was fractionated by step elution on DEAE-Sepharose and Biorex 70, followed by linear gradient elution from Mono Q using FPLC. Protein concentration and nonspecific polymerase activity in the presence of 100 μg/ml α-amanitin was measured for each fraction. One unit of pol activity is defined as 1,000 cpm incorporated in the nonspecific assay (see Materials and Methods). (B) Elution profile of total protein and nonspecific polymerase activity on Mono Q using the fractionation scheme depicted in A. The column was eluted with a 10-column volume linear gradient from 0.1 M to 0.6 M KCl. Eluted proteins were monitored continuously by UV absorbance. Fractions 10–20 were assayed for nonspecific polymerase activity in the presence of 100 μg/ml α-amanitin (dashed line). (C) Single Mono Q fractions program accurate transcription initiation from the B. oleracea rRNA gene promoter. Fractions 9–19 were mixed with 500 ng of pBor2 in reactions containing 100 μg/ml α-amanitin. Transcripts were detected by S1 nuclease protection using a probe that spanned the transcription start site (see diagram). The labeled XbaI site is derived from the plasmid adjacent to the cloned promoter sequences thus the probe only detects transcripts derived from the plasmid. Dideoxynucleotide sequencing reactions, using a primer end-labeled at the same nucleotide as the S1 probe, were run in adjacent lanes to verify initiation at the in vivo start site TATATAAGGG (+1 is underlined).
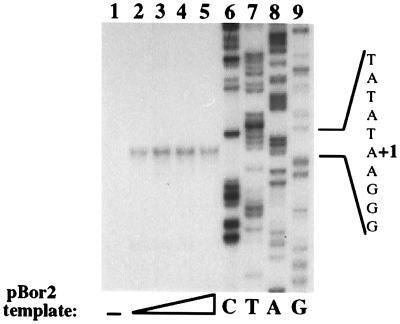
Figure 2.
Accurate transcription initiation programmed by peak Mono Q fractions is relatively insensitive to template concentration. Totals of 125, 250, 500, or 1,000 ng (lanes 2–5, respectively) of pBor2 plasmid DNA was added to transcription reactions containing the peak Mono Q fraction (no. 16) in the presence of 100 μg/ml α-amanitin. In lane 1, plasmid DNA not containing an rRNA gene promoter was present in an equivalent reaction. A dideoxy sequencing ladder was generated and run beside the reactions as in Fig. 1.
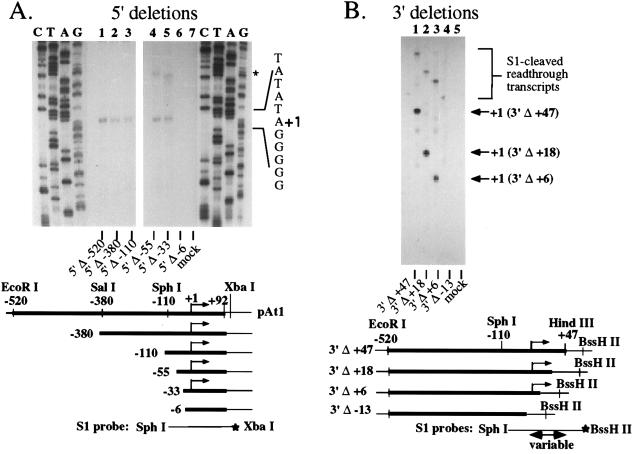
Figure 3.
rRNA gene promoter sequences between −33 and +6 are required for transcription in vitro. A series of 5′ (A) and 3′ (B) A. thaliana promoter deletion constructs derived from pAt1 were tested in vitro using the peak Mono Q fraction (purified according to Fig. 1) in the presence of 100 μg/ml α-amanitin. A total of 500 ng of each plasmid was tested, and transcripts were detected by S1 protection using the probes shown at the bottom of the figures. Different probes had to be used for each 3′ deletion construct; thus the protected fragments are different sizes. Reactions labeled “mock” were performed using Mono Q fractionated protein but no template.
In the four-column purification scheme, the Biorex 800 fraction (in ≈3 ml) was loaded directly onto a 195 ml Sephacryl S-300 (Pharmacia) FPLC column (≈97 cm long) equilibrated and run in CB100. Peak polymerase-containing fractions were subjected to Mono Q chromatography as described above.
DNA Templates and in Vitro Transcription Reactions.
Plasmid pBor2 contains B. oleracea rRNA gene promoter sequences from −518 to +106 (26). The Arabidopsis thaliana rRNA gene promoter clone pAt1 contains sequences from −520 to +92. pAt1 and its deletion derivatives have been described (24, 25). For in vitro transcription reactions, 20 μl of Mono Q purified protein was mixed with 125–1,000 ng (see figure legends) of supercoiled pBor2 or pAt1 plasmid DNA. Following a 5-min incubation at room temperature, 20 μl of 2× reaction mixture (30 mM Hepes, pH 7.9/80 mM potassium acetate/12 mM magnesium acetate/1 mM DTT/200 μg/ml α-amanitin/1 mM of each nucleotide triphosphate) was added. Transcription reactions were incubated 2 h at 25°C and terminated by addition of 360 μl of stop solution [150 mM NaCl/50 mM Tris⋅HCl, pH 8.0/250 mM sodium acetate, pH 5.3/3 μg/ml yeast tRNA/6 mM EDTA, pH 8.0] followed by extraction with an equal volume of phenol/chloroform and vortex mixing. Following brief centrifugation in a microcentrifuge, the aqueous phase was mixed with ≈2 × 104 cpm of probe DNA (see figures) 5′ end-labeled with T4 polynucleotide kinase and γ-labeled [32P]ATP (34). RNA transcripts and probe were coprecipitated with ethanol and resuspended in 30 μl of hybridization buffer (80% formamide/40 mM Pipes, pH 6.4/400 mM NaCl/1 mM EDTA). After initial denaturation for 10 min at 100°C, hybridization was carried out at 37°C overnight. A total of 270 μl of S1 digestion buffer (34) containing 125 units/ml S1 nuclease (Sigma) was added. S1 digestion reactions were incubated 30 min at 37°C and stopped by addition of 5 μl 0.5 M EDTA. Following addition of 30 μl 7.5 M ammonium acetate, reaction products were ethanol precipitated. Following centrifugation, pellets were resuspended in 95% formamide loading dye and subjected to electrophoresis on 6% polyacrylamide/7 M urea sequencing gels (34). Digestion products were visualized by autoradiography or by a PhosphorImager (Molecular Dynamics).
RESULTS
Development of a Plant in Vitro Transcription System for RNA Pol I.
Broccoli inflorescence (the part we generally eat) is an inexpensive source of rapidly dividing cells in which pol I transcription is expected to be very active. Soluble proteins extracted from crude nuclei were precipitated, dialyzed into 100 mM KCl buffer (RB100), and purified first by step fractionation on a DEAE-Sepharose anion-exchange column (Fig. 1A). Approximately 15% of total α-amanitin-resistant nonspecific polymerase activity (assayed on nicked calf thymus DNA) flowed through the DEAE column in RB100. An additional 35% was eluted with RB200, followed by a final ≈50% that eluted with RB400 (DEAE 400 fraction). RNA pol III in Brassica is highly resistant to α-amanitin and is inhibited <50% even at 1 mg/ml (33). Thus the contributions of pol I and pol III to the activity detected in DEAE fractions is not discriminated in the nonspecific assay. However, the DEAE 400 fraction, which accounted for 13% of the starting amanitin-resistant polymerase activity, programmed weak transcription from the in vivo start site using the B. oleracea rRNA gene promoter construct pBor2 (data not shown). DEAE 100 and DEAE 200 fractions lacked comparable activity. Combining DEAE fractions did not further stimulate nonspecific or promoter-dependent transcription.
Following dialysis against RB100, the DEAE 400 fraction was loaded onto a Biorex 70 cation-exchange column equilibrated in RB100. Greater than 90% of the nonspecific polymerase activity was retained on the column and was eluted in a single step with RB800; the remaining ≈10% flowed through the column. The yield of polymerase activity in the Biorex 800 fraction was ≈3% of the starting activity (Fig. 1A).
Following dialysis against CB100, the Biorex 800 fraction was subjected to chromatography on an analytical Mono Q anion exchange column and eluted with a 20 ml linear salt gradient from CB100 to CB600 (Fig. 1B). Individual fractions were tested for nonspecific pol I activity and the ability to program promoter-dependent pol I transcription. Nonspecific pol I activity eluted at ≈450 mM KCl and peaked in fractions 14–16. These same fractions initiated transcripts from the B. oleracea rRNA gene promoter, with fraction 16 representing the peak of promoter-dependent activity (Fig. 1C). Transcription signals remained fairly uniform using a broad range of template concentrations and did not display template inhibition at any concentration tested (Fig. 2 and data not shown).
The Brassica Pol I-Containing Protein Complex Transcribes the Arabidopsis Promoter and Requires the Same Promoter Sequences Needed in Vivo.
The B. oleracea promoter is active when transfected into cells of A. thaliana, a related species (26). Therefore, we expected an A. thaliana rRNA gene promoter to be transcribed in the broccoli in vitro system, allowing promoter deletion mutants already characterized by transient expression (24, 25) to be used to compare promoter requirements in vitro and in vivo. As predicted, transcripts were accurately initiated from the Arabidopsis promoter construct pAt1 (Fig. 3A, lane 1). The Brassica promoter was only slightly stronger than the pAt1 promoter construct in side-by-side reactions (less than 2-fold; data not shown).
In vivo, the upstream boundary of the A. thaliana rRNA gene promoter was shown to be between −55 and −33 and is probably closest to −33 (25). This conclusion is based on four transient expression experiments in which a −55 (pAt1 5′Δ-55) deletion construct was always functional. In two trials, weak expression was detected from a promoter deleted to −33. However, in the other two experiments, transcripts from the 5′Δ-33 construct were not detected. The downstream promoter boundary was mapped by transient expression using constructs whose 5′ boundary was −520, but whose 3′ sequences were sequentially deleted (25). Deletion to +6 had no effect on promoter activity, but further deletion to positions upstream of the transcription start site abolished transcription. We used these same 5′ and 3′ deletion constructs to test the promoter sequences required in vitro with the peak Mono Q fraction purified as in Fig. 1. Deletion of 5′ sequences from −520 to −380, −110, −55, or −33 all yielded similar levels of accurately initiated transcripts in vitro (Fig. 3A, lanes 1–5). Further deletion to −6 abolished transcription (lane 6), indicating that the 5′ promoter boundary in vitro is between −33 and −6. Likewise, 3′ deletions to +47, +18, or +6 were fully functional for transcription (Fig. 3B; lanes 1–3, respectively), but further deletion to −13 abolished transcription (lane 4). Read-through transcripts detected in Fig. A (see lanes 4 and 5 in the region denoted by an asterisk) and B are due to pol I transcripts that traverse the promoter region from upstream due to the use of circular plasmid templates.
The results of Fig. 3 agree closely with those obtained in vivo. The major difference was that the 5′Δ-33 construct was expressed at approximately the same level as the −55 construct in vitro whereas in vivo, the −33 construct was only weakly expressed, at best. A parallel is that in mammalian systems, core promoter sequences are often sufficient to program transcription in vitro, but additional upstream sequences are needed for efficient transcription in vivo (35–38).
Purification of the Putative Pol I Holoenzyme to Near-Homogeneity.
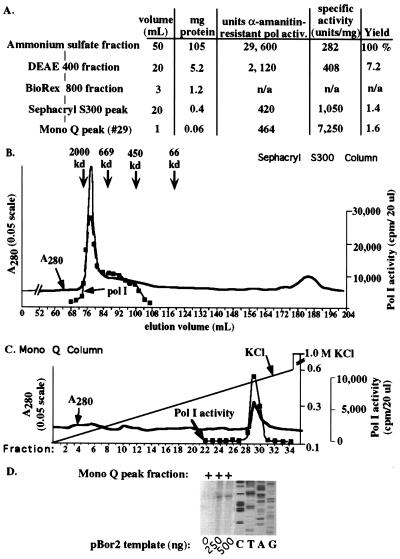
The experiments above suggested that the Mono Q fractions contain all activities required for core promoter recognition and transcription initiation. If these activities were associated within a single complex, we reasoned that they should also copurify when fractionated by size. Therefore, we subjected an undialyzed ≈3 ml Biorex 800 fraction to gel filtration chromatography using a 195 ml Sephacryl-S300 column equilibrated and run in CB100 (Fig. 4A). Nonspecific pol I activity, and a significant portion of the total protein, eluted as a sharp peak followed by a trailing shoulder of activity (Fig. 4B). The major peak eluted with the trailing edge of Blue Dextran, which has an average molecular weight of 2 MDa. A standard curve generated by plotting relative elution volume versus the log of the molecular mass for Blue Dextran (2 MDa assumed as the mass of the peak), thyroglobulin (669 kDa), ferritin (450 kDa), and BSA led to an estimated mass of ≈1.7 MDa ± ≈0.3 MDa) for the pol I-containing peak. The trailing shoulder of pol I activity tailed off at ≈500 kDa, the approximate size of the pol I core enzyme (39).
Figure 4.
Purification of the putative B. oleracea pol I holoenzyme to near homogeneity. (A) Fractionation scheme and summary data. Following DEAE and Biorex chromatography, the Biorex 800 fraction was loaded directly onto a 195 ml Sephacryl S300 column equilibrated and run in 100 mM KCl buffer (see B). Peak pol I-containing fractions were then subjected to Mono Q chromatography (see C). (B) Elution profile of total protein and nonspecific pol I activity on Sephacryl S300. Proteins were detected by UV absorbance at 280 nm. Individual fractions were then tested for α-amanitin insensitive nonspecific transcription. Peak elution positions for Blue Dextran, thyroglobulin, ferritin, and BSA molecular mass standards are indicated. (C) Mono Q chromatography of Sephacryl peak fractions was performed as in Fig. 1. Fractions were dialyzed and tested for α-amanitin-resistant polymerase activity. The major UV-absorbing protein peak corresponded to the peak of polymerase activity. (D) Peak Mono Q fractions program accurate transcription initiation. Aliquots of the Mono Q peak were added to transcription reactions containing 0, 250, or 500 ng of pBor2 plasmid DNA in the presence of 100 μg/ml α-amanitin. Transcripts were detected by S1 nuclease protection.
Peak pol I fractions from the Sephacryl column were pooled and subjected to chromatography on Mono Q. Elution of the Mono Q column as in Fig. 1 led to a single major UV-absorbing peak eluting at ≈450 mM KCl (Fig. 4C). The peak of nonspecific pol I activity corresponded to precisely these same fractions (Fig. 4C), as did promoter-dependent pol I transcription activity (Fig. 4D).
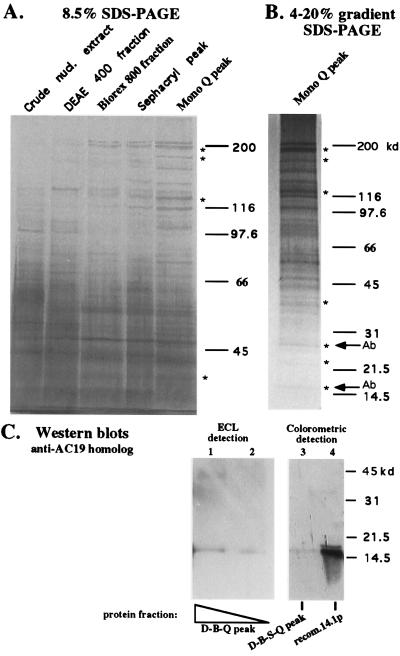
The fact that the pol I activity eluted from Mono Q with the only major protein peak suggested that the complex was purified to near-homogeneity. Therefore peak fractions throughout the purification scheme (see Fig. 4A) were compared qualitatively (Fig. 5A). The polypeptide composition of the last two peak fractions (Sephacryl and Mono Q) was very similar, suggesting that the complex was approaching purity (at which point no further purification is achieved by subsequent steps). Approximately 30 distinct protein bands are apparent in the final peak Mono Q fraction, resolved best on a 4–20% gradient SDS/polyacrylamide gel (Fig. 5B). These include proteins of the appropriate size to be subunits of pol I core enzyme, whose expected positions are denoted by asterisks in Fig. 5. Two of these, migrating at ≈15 kDa and 27 kDa, were confirmed as polymerase subunits by Western blot analysis using antibodies generously provided by Robert Larkin and Tom Guilfoyle (University of Missouri). Antisera against the 14.1 kDa (predicted) Arabidopsis homolog of the yeast pol I/pol III subunit AC19 (40) cross-reacted with a protein of the appropriate size in Mono Q fractions (Fig. 5C) purified according to the three column procedure diagrammed in Fig. 1A (lanes 1 and 2) or the four column procedure of Fig. 4 (lane 3). The Western blot analysis result with the anti-27-kDa subunit antibody is not shown because Larkin and Guilfoyle have not yet published the cloning and characterization of this subunit.
Figure 5.
Polypeptide composition of highly purified holoenzyme fractions. (A) Equal amounts of protein from crude nuclear extract, the DEAE 400 mM KCl fraction, Sephacryl S300 peak, and Mono Q peak fractions were subjected to electrophoresis on an 8.5% SDS/polyacrylamide gel and visualized by silver staining. (B) The Mono Q peak fraction was also subjected to electrophoresis on a 4–20% gradient SDS gel. Asterisks indicate the expected sizes for subunits of the pol I core enzyme based on the work of Guilfoyle (39). Arrows labeled Ab indicate polymerase subunits verified by Western blot analysis (C and data not shown). (C) Western blot analysis using an antibody against the Arabidopsis homolog of the yeast AC19 polymerase subunit. Lanes: 1 and 2, Mono Q peak fraction (two amounts) following DEAE and Biorex (D-B-Q fractions; see Fig. 1A); 3, Mono Q peak fraction after the four column scheme of Fig. 4A (D-B-S-Q; lane 3); 4, 30 ng of the purified recombinant 14.1-kDa (predicted size) protein used to raise the antibodies (gift of R. Larkin and T. Guilfoyle). Enhanced chemiluminescence detection was used in lanes 1 and 2, and colorimetric detection was used in lanes 3 and 4. Proteins were resolved on a 12.5% SDS/PAGE gel prior to blotting to nitrocellulose. Pre-immune serum did not cross-react on duplicate blots (data not shown).
Though silver-staining intensity is often variable between proteins, the major bands apparent in Fig. 5B appear to be comparable in abundance to the small polymerase subunits verified by Western blot analysis. Minor bands may be breakdown products of larger proteins, proteins associated with polymerase in substoichiometric amounts, proteins that do not stain well with silver, or contaminating proteins that copurify with RNA pol I on all four columns.
DISCUSSION
We have developed a cell-free transcription system from broccoli (B. oleracea) that is α-amanitin insensitive, initiates transcription from the proper start site, and requires the same promoter regions needed in vivo. These initial results suggested that our fractions contained an activity that could account for the known attributes of pol I transcription in vivo. Subsequent purification of transcriptionally active fractions by DEAE, Biorex, gel filtration, and Mono Q chromatography yielded a single major protein peak on the final column, an indication of purity or near-purity. Approximately 30 distinct polypeptides are present in such peak fractions, two of which cross-react with the only two currently available antibodies against proteins expected to be plant pol I subunits. The sum of the masses of the ≈30 protein bands is ≈2 MDa, in reasonable agreement with the ≈1.7 MDa estimate from Sephacryl chromatography. Thirty proteins are also a plausible complexity for the pol I transcription system. In mouse, pol I core enzyme (11 subunits) (41), SL1/TIF-IB (4 subunits), UBF, TIF-IA, and TIF-IC account for at least 18 proteins. In yeast, 24 known proteins participate in pol I transcription: the pol I core enzyme is composed of 14 subunits (42), a complex of 4 proteins recognizes the core promoter (TBP, Rrn proteins 6, 7, and 11) (43, 44), a complex of 5 proteins interacts with the upstream promoter domain (Rrn proteins 5, 9 and 10 and two unidentified proteins) (45), and Rrn3p interacts with the polymerase core enzyme in solution (46).
The fact that the DEAE 400 fraction programmed accurate transcription from the Brassica promoter was not unexpected because a similar DEAE fraction programs in vitro pol I transcription in yeast, Xenopus, and mammals (15, 47, 48). Subsequent copurification of all necessary factors on Biorex, Sephacryl and Mono Q was more surprising, but is also true in Xenopus (A.-C. Albert, M. Denton, and C.S.P., unpublished work). Glycerol gradient sedimentation of crude or partially purified Xenopus cell extracts also results in single fractions capable of promoter-dependent transcription (M. Denton and C.S.P., unpublished work). It seems unlikely that all essential transcription factors copurify with polymerase by virtue of having the same elution characteristics on multiple anion exchange, cation exchange, and gel filtration columns. Instead, the simplest explanation is that these activities are associated, comprising a holoenzyme complex. We point out that a protein complex, known as a mediator, can be separated from the pol II holoenzyme under certain conditions (49); thus the ability to fractionate a transcription system into multiple activities does not show definitively that the activities do not associate as metastable complexes in the cell.
Polymerase activity in different extracts of store-bought broccoli is variable (see Figs. 1A and 4A) but does not affect the purification scheme. More difficult to interpret is the yield of pol I activity and what this implies about the abundance of the putative holoenzyme. Yields of 1–2% (Figs. 1A and 4A) are slight underestimates given that both pol I and pol III contribute to amanitin-resistant transcription in starting extracts, but such yields are not unreasonable. In human cells, it is estimated that there are several hundred copies of SL1, but 104–105 pol I molecules (16). Likewise in yeast, core factor and upstream activation factor complexes appear to be present in hundreds of copies per cell whereas polymerase is estimated at ≈104 copies (45). These estimates suggests that no more than 2–10% of pol I could be associated with transcription factors to form holoenzyme complexes, in reasonable agreement with our data.
Without knowing the exact ratio of free polymerase to holoenzyme, it is difficult to estimate the degree of purification we have achieved. Based on initial α-amanitin resistant polymerase activity, purification of the holoenzyme to near-homogeneity results in only a 26-fold increase in polymerase specific activity (Fig. 4A). However, if only 5–10% of the starting polymerase activity were pol I holoenzyme (and 90–95% were pol III and pol I core enzyme), the degree of holoenzyme purification would be 260- to 520-fold. We also expect ≈3- to 4-fold lower polymerase activity per microgram of protein for holoenzyme versus core polymerase due to the larger mass of the former. Furthermore, less polymerase activity is recovered than is loaded onto each column, even when fractions are recombined, indicating that polymerase activity decays throughout purification. Such lability negatively affects specific activity and thus the estimated degree of purification. We anticipate that antibodies specific for plant pol I subunits, currently unavailable, should allow us to better follow pol I throughout purification and better estimate the abundance of the putative holoenzyme.
Acknowledgments
We are grateful to Rob Larkin and Tom Guilfoyle (University of Missouri) for generously providing antibodies against recombinant A. thaliana RNA polymerase subunits and for sharing unpublished results. We thank our fellow lab members Annie-Claude Albert, L. Annie Shen, and Milko Kermekchiev for their help and input. This work was supported by grants to C.S.P. from the NRI Competitive Grants Program/U.S. Department of Agriculture (Grant 94-373012-0658) and the National Institutes of Health (Grant GM50910-03). J.S.-V. was supported in part by a Monsanto Postdoctoral Fellowship in Plant Biology.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: pol I, II, and III, RNA polymerase I, II, and III; FPLC, fast protein liquid chromatography.
References
- 1.Zawel L, Reinberg D. Prog Nucleic Acid Res Mol Biol. 1993;44:67–108. doi: 10.1016/s0079-6603(08)60217-2. [DOI] [PubMed] [Google Scholar]
- 2.Roeder R. Trends Biochem Sci. 1991;16:402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- 3.Serizawa H, Conaway J W, Conaway R C. In: Transcription: Mechanisms and Regulation. Conaway R C, Conaway J W, editors. New York: Raven; 1994. pp. 27–44. [Google Scholar]
- 4.Kassavetis G A, Bardeleben C, Bartholomew B, Braun B R, Joazeiro C A P, Pisano M, Geiduschek E P. In: Transcription: Mechanisms and Regulation. Conaway R C, Conaway J W, editors. New York: Raven; 1994. pp. 107–126. [Google Scholar]
- 5.Paule M R. In: Transcription: Mechanisms and Regulation. Conaway R C, Conaway J W, editors. New York: Raven; 1994. pp. 83–106. [Google Scholar]
- 6.Moss T, Stefanovsky V Y. Prog Nucleic Acid Res Mol Biol. 1995;50:25–66. doi: 10.1016/s0079-6603(08)60810-7. [DOI] [PubMed] [Google Scholar]
- 7.Orphanides G, Lagrange T, Reinberg D. Genes Dev. 1996;10:2657–2683. doi: 10.1101/gad.10.21.2657. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Bjorklund S, Kim Y J, Kornberg R D. Methods Enzymol. 1996;273:172–175. doi: 10.1016/s0076-6879(96)73017-3. [DOI] [PubMed] [Google Scholar]
- 9.Ossipow V, Tassan J P, Nigg E A, Schibler U. Cell. 1995;83:137–146. doi: 10.1016/0092-8674(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 10.Koleske A J, Young R A. Trends Biochem Sci. 1995;20:113–116. doi: 10.1016/s0968-0004(00)88977-x. [DOI] [PubMed] [Google Scholar]
- 11.Koleske A J, Young R A. Nature (London) 1994;368:466–469. doi: 10.1038/368466a0. [DOI] [PubMed] [Google Scholar]
- 12.Maldonado E, Shiekhattar R, Sheldon M, Cho H, Drapkin R, Rickert P, Lees E, Anderson C W, Linn S, Reinberg D. Nature (London) 1996;381:86–89. doi: 10.1038/381086a0. [DOI] [PubMed] [Google Scholar]
- 13.Liao S M, Zhang J, Jeffery D A, Koleske A J, Thompson C M, Chao D M, Viljoen M, vanVuuren H J, Young R A. Nature (London) 1995;374:193–196. doi: 10.1038/374193a0. [DOI] [PubMed] [Google Scholar]
- 14.Wilson C J, Chao D M, Imbalzano A N, Schnitzler G R, Kingston R E, Young R A. Cell. 1996;84:235–244. doi: 10.1016/s0092-8674(00)80978-2. [DOI] [PubMed] [Google Scholar]
- 15.Kurl R N, Rothblum L I, Jacob S T. Proc Natl Acad Sci USA. 1984;81:6672–6675. doi: 10.1073/pnas.81.21.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell S P, Learned R M, Jantzen H M, Tjian R. Science. 1988;241:1192–1197. doi: 10.1126/science.3413483. [DOI] [PubMed] [Google Scholar]
- 17.Hempel W M, Cavanaugh A H, Hannan R D, Taylor L, Rothblum L I. Mol Cell Biol. 1996;16:557–563. doi: 10.1128/mcb.16.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodeker M, Cairns C, McStay B. Mol Cell Biol. 1996;16:5572–5578. doi: 10.1128/mcb.16.10.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnapp G, Santori F, Carles C, Riva M, Grummt I. EMBO J. 1994;13:190–199. doi: 10.1002/j.1460-2075.1994.tb06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnapp A, Pfleiderer C, Rosenbauer H, Grummt I. EMBO J. 1990;9:2857–2863. doi: 10.1002/j.1460-2075.1990.tb07475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnapp G, Schnapp A, Rosenbauer H, Grummt I. EMBO J. 1994;13:4028–4035. doi: 10.1002/j.1460-2075.1994.tb06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita J, Nakajima T, Tanifuji S, Kato A. Plant J. 1993;3:187–190. [Google Scholar]
- 23.Fan H, Yakura K, Miyanishi M, Sugita M, Sugiura M. Plant J. 1995;8:295–298. doi: 10.1046/j.1365-313x.1995.08020295.x. [DOI] [PubMed] [Google Scholar]
- 24.Doelling J H, Gaudino R J, Pikaard C S. Proc Natl Acad Sci USA. 1993;90:7528–7532. doi: 10.1073/pnas.90.16.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doelling J H, Pikaard C S. Plant J. 1995;8:683–692. doi: 10.1046/j.1365-313x.1995.08050683.x. [DOI] [PubMed] [Google Scholar]
- 26.Doelling J H, Pikaard C S. Nucleic Acids Res. 1996;24:4725–4732. doi: 10.1093/nar/24.23.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaudino R J, Pikaard C S. J Biol Chem. 1997;272:6799–6804. doi: 10.1074/jbc.272.10.6799. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z J, Pikaard C S. Proc Natl Acad Sci USA. 1997;94:3442–3447. doi: 10.1073/pnas.94.7.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieber J J, Lopez M F, Tissier A F, Signer E. Plant Mol Biol. 1992;18:865–871. doi: 10.1007/BF00019201. [DOI] [PubMed] [Google Scholar]
- 30.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Roeder R G. J Biol Chem. 1983;258:1932–1941. [PubMed] [Google Scholar]
- 32.Engelke D R, Shastry B S, Roeder R G. J Biol Chem. 1983;10:1921–1931. [PubMed] [Google Scholar]
- 33.Guilfoyle T J. Plant Physiol. 1976;58:453–458. doi: 10.1104/pp.58.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: a Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 35.Clos J, Normann A, Ohrlein A, Grummt I. Nucleic Acids Res. 1986;14:7581–7595. doi: 10.1093/nar/14.19.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haltiner M M, Smale S T, Tjian R. Mol Cell Biol. 1986;6:227–235. doi: 10.1128/mcb.6.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones M H, Learned R M, Tjian R. Proc Natl Acad Sci USA. 1988;85:669–673. doi: 10.1073/pnas.85.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henderson S L, Sollner-Webb B. Mol Cell Biol. 1990;10:4970–4973. doi: 10.1128/mcb.10.9.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guilfoyle T J. Biochemistry. 1980;19:5966–5972. doi: 10.1021/bi00567a004. [DOI] [PubMed] [Google Scholar]
- 40.Larkin R M, Guilfoyle T J. Gene. 1996;172:211–215. doi: 10.1016/0378-1119(96)00030-3. [DOI] [PubMed] [Google Scholar]
- 41.Song C Z, Hanada K, Yano K, Maeda Y, Yamamoto K, Muramatsu M. J Biol Chem. 1994;269:26976–26981. [PubMed] [Google Scholar]
- 42.Sentenac A. Crit Rev Biochem. 1985;18:31–91. doi: 10.3109/10409238509082539. [DOI] [PubMed] [Google Scholar]
- 43.Lalo D, Steffan J S, Dodd J A, Nomura M. J Biol Chem. 1996;271:21062–21067. doi: 10.1074/jbc.271.35.21062. [DOI] [PubMed] [Google Scholar]
- 44.Lin C W, Moorefield B, Payne J, Aprikian P, Mitomo K, Reeder R H. Mol Cell Biol. 1996;16:6436–6443. doi: 10.1128/mcb.16.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keys D A, Lee B S, Dodd J A, Nguyen T T, Vu L, Fantino E, Burson L M, Nogi Y, Nomura M. Genes Dev. 1996;10:887–903. doi: 10.1101/gad.10.7.887. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto R T, Nogi Y, Dodd J A, Nomura M. EMBO J. 1996;15:3964–3973. [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz M C, Choe S Y, Reeder R H. Proc Natl Acad Sci USA. 1991;88:1004–1008. doi: 10.1073/pnas.88.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McStay B, Reeder R H. Cell. 1986;47:913–920. doi: 10.1016/0092-8674(86)90806-8. [DOI] [PubMed] [Google Scholar]
- 49.Kim Y J, B S, Li Y, Sayre M H, Kornberg R D. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]