Abstract
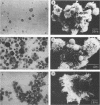
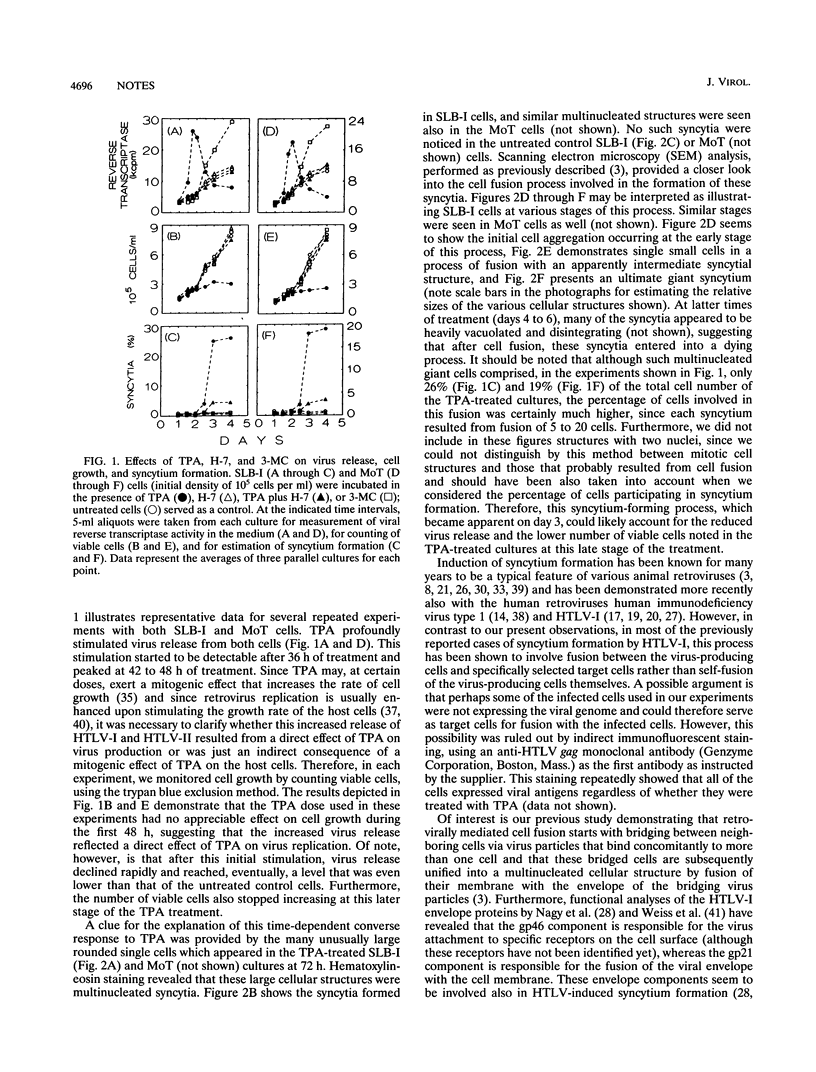
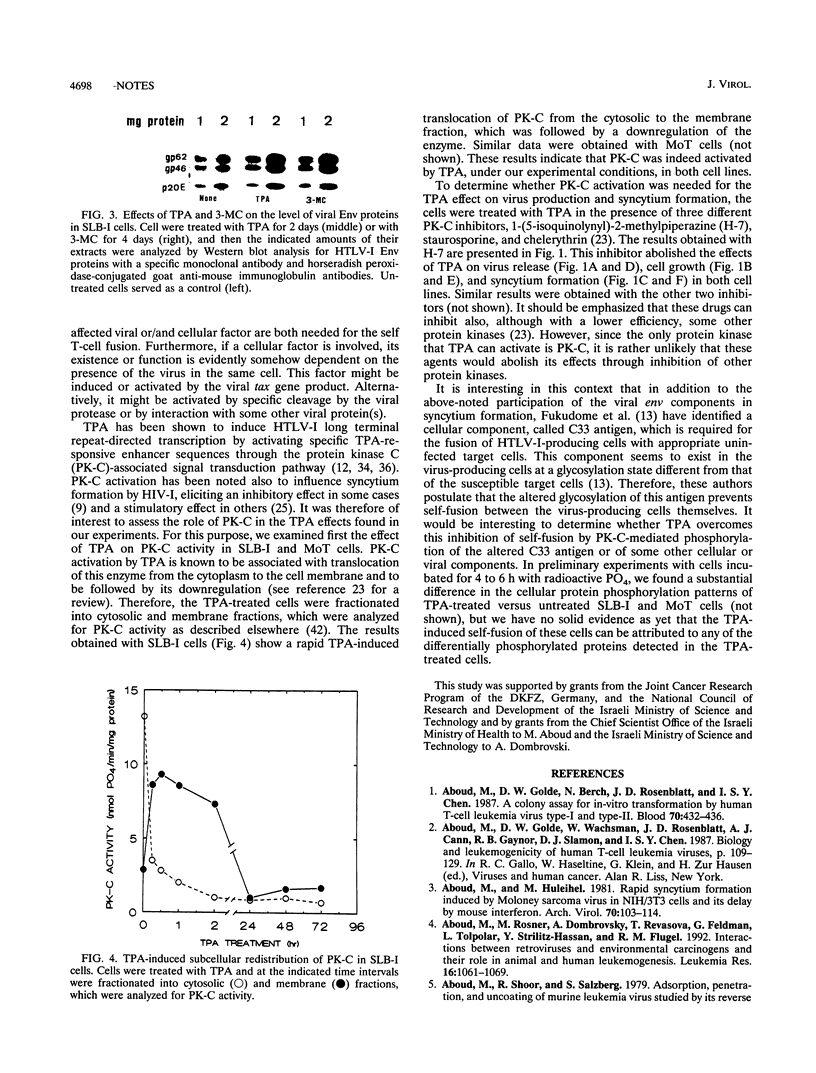
Treatment of human T-cell leukemia virus type I (HTLV-I)- and HTLV-II-infected T-cell lines with 12-O-tetradecanoylphorbol-13-acetate (TPA) stimulated virus release. However, this stimulation was mainly detected at 42 to 48 h of treatment, whereas later virus release declined rapidly. During the first 48 h, TPA had no effect on cell growth, but later, the number of viable cells was profoundly lower in the TPA-treated than in the untreated cultures. This shift in virus release and cell number resulted from self-fusion of a large proportion of the virus-producing cells, which seemed to consequently enter into a dying process. This fusion, which resulted in syncytium formation, was strongly inhibited by anti-HTLV-I env monoclonal antibodies. Furthermore, no self-fusion was detected in three different uninfected T-cell lines similarly treated with TPA. On the other hand, stimulation of virus production by 3-methylcholanthrene (3-MC) treatment failed to induce self-fusion in the infected cells. Moreover, no syncytium was detected when these 3-MC-treated infected cells were cocultured with any of the TPA-treated uninfected cells. The effects of TPA on virus production and syncytium formation were both abolished by three different protein kinase C inhibitors. Taken together, these data suggest that the self-fusion observed in these experiments required both enhanced virus production and protein kinase C-phosphorylated viral or/and virally induced cellular component(s).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboud M., Golde D. W., Bersch N., Rosenblatt J. D., Chen I. S. A colony assay for in vitro transformation by human T cell leukemia viruses type I and type II. Blood. 1987 Aug;70(2):432–436. [PubMed] [Google Scholar]
- Aboud M., Huleihel M. Rapid syncytium formation induced by Moloney murine sarcoma virus in 3T3/NIH cells and its delay by mouse interferon. Arch Virol. 1981;70(2):103–114. doi: 10.1007/BF01315004. [DOI] [PubMed] [Google Scholar]
- Aboud M., Rosner M., Dombrovsky A., Revazova T., Feldman G., Tolpolar L., Strilitz-Hassan Y., Flügel R. M. Interactions between retroviruses and environmental carcinogens and their role in animal and human leukemogenesis. Leuk Res. 1992 Nov;16(11):1061–1069. doi: 10.1016/0145-2126(92)90044-8. [DOI] [PubMed] [Google Scholar]
- Aboud M., Shoor R., Salzberg S. Adsorption, penetration, and uncoating of murine leukemia virus studied by using its reverse transcriptase. J Virol. 1979 Apr;30(1):32–37. doi: 10.1128/jvi.30.1.32-37.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L. J., Blakeslee J. R., Jr The effects of tumor promoters on HTLV-1 expression in a low-virus producer and a non-virus producer cell line. Cancer Lett. 1988 Apr;39(3):329–338. doi: 10.1016/0304-3835(88)90077-8. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Hunter E. Inhibition of Mason-Pfizer monkey virus-induced syncytium formation in normal human cells by homologous interferon. Virology. 1980 Jul 30;104(2):487–490. doi: 10.1016/0042-6822(80)90351-7. [DOI] [PubMed] [Google Scholar]
- Chowdhury I. H., Koyanagi Y., Kobayashi S., Hamamoto Y., Yoshiyama H., Yoshida T., Yamamoto N. The phorbol ester TPA strongly inhibits HIV-1-induced syncytia formation but enhances virus production: possible involvement of protein kinase C pathway. Virology. 1990 May;176(1):126–132. doi: 10.1016/0042-6822(90)90237-l. [DOI] [PubMed] [Google Scholar]
- Fujii M., Nakamura M., Ohtani K., Sugamura K., Hinuma Y. 12-O-tetradecanoylphorbol-13-acetate induces the enhancer function of human T-cell leukemia virus type I. FEBS Lett. 1987 Nov 2;223(2):299–303. doi: 10.1016/0014-5793(87)80308-3. [DOI] [PubMed] [Google Scholar]
- Fujisawa J., Toita M., Yoshida M. A unique enhancer element for the trans activator (p40tax) of human T-cell leukemia virus type I that is distinct from cyclic AMP- and 12-O-tetradecanoylphorbol-13-acetate-responsive elements. J Virol. 1989 Aug;63(8):3234–3239. doi: 10.1128/jvi.63.8.3234-3239.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukudome K., Furuse M., Imai T., Nishimura M., Takagi S., Hinuma Y., Yoshie O. Identification of membrane antigen C33 recognized by monoclonal antibodies inhibitory to human T-cell leukemia virus type 1 (HTLV-1)-induced syncytium formation: altered glycosylation of C33 antigen in HTLV-1-positive T cells. J Virol. 1992 Mar;66(3):1394–1401. doi: 10.1128/jvi.66.3.1394-1401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth J. E., Orentas R. J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989 Jun 2;244(4908):1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of human endothelial cells by human T-lymphotropic virus type I. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7588–7590. doi: 10.1073/pnas.81.23.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. J., Luethy J. D., Kin J. Transient expression of foreign genes in lymphoid cells is enhanced by phorbol ester. Mol Cell Biol. 1987 Jul;7(7):2610–2613. doi: 10.1128/mcb.7.7.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino H., Shimoyama M., Miwa M., Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7337–7341. doi: 10.1073/pnas.80.23.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie J. A., Matthews D. M., Cines D. B. Infection of human endothelial cells by human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7591–7595. doi: 10.1073/pnas.81.23.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irgens K., Pinelli C., Guillemain B., Levy D., Parodi A. L. Early syncytium formation induced by bovine leukemia virus in mixed cultures. Biomedicine. 1977 Mar;27(2):49–50. [PubMed] [Google Scholar]
- Jaken S. Protein kinase C and tumor promoters. Curr Opin Cell Biol. 1990 Apr;2(2):192–197. doi: 10.1016/0955-0674(90)90006-z. [DOI] [PubMed] [Google Scholar]
- MOSES E., KOHN A. POLYKARYOCYTOSIS INDUCED BY ROUS SARCOMA VIRUS IN CHICK FIBROBLASTS. Exp Cell Res. 1963 Oct;32:182–186. doi: 10.1016/0014-4827(63)90086-7. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Nakao Y., Ohigashi H., Koshimizu K., Ito Y. Plant-derived diterpene esters enhance HTLV-I-induced colony formation of lymphocytes in co-culture. Int J Cancer. 1986 Dec 15;38(6):859–865. doi: 10.1002/ijc.2910380613. [DOI] [PubMed] [Google Scholar]
- Mohagheghpour N., Chakrabarti R., Stein B. S., Gowda S. D., Engleman E. G. Early activation events render T cells susceptible to HIV-1-induced syncytia formation. Role of protein kinase C. J Biol Chem. 1991 Apr 15;266(11):7233–7238. [PubMed] [Google Scholar]
- Nagy K., Clapham P., Cheingsong-Popov R., Weiss R. A. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients' sera. Int J Cancer. 1983 Sep 15;32(3):321–328. doi: 10.1002/ijc.2910320310. [DOI] [PubMed] [Google Scholar]
- Nakao Y., Matsuda S., Matsui T., Nakagawa T., Fujita T., Uchiyama T., Maeda S., Okamoto Y., Masaoka T., Ito Y. Effect of tumor promoters on human T cell leukemia/lymphoma virus (HTLV)-structural protein induction in adult T-cell leukemia cells. Cancer Lett. 1984 Sep;24(2):129–139. doi: 10.1016/0304-3835(84)90128-9. [DOI] [PubMed] [Google Scholar]
- Noronha F., Dougherty E., Poco A., Gries C., Post J., Rickard C. Cytological and serological studies of a feline endogenous C-type virus. Arch Gesamte Virusforsch. 1974;45(3):235–248. doi: 10.1007/BF01249686. [DOI] [PubMed] [Google Scholar]
- Numata N., Ohtani K., Niki M., Nakamura M., Sugamura K. Synergism between two distinct elements of the HTLV-I enhancer during activation by the trans-activator of HTLV-I. New Biol. 1991 Sep;3(9):896–906. [PubMed] [Google Scholar]
- Ogura H. XC cell fusion by murine leukemia viruses: fusion from without. Med Microbiol Immunol. 1976 Dec 1;162(3-4):175–181. doi: 10.1007/BF02120995. [DOI] [PubMed] [Google Scholar]
- Radonovich M., Jeang K. T. Activation of the human T-cell leukemia virus type I long terminal repeat by 12-O-tetradecanoylphorbol-13-acetate and by tax (p40x) occurs through similar but functionally distinct target sequences. J Virol. 1989 Jul;63(7):2987–2994. doi: 10.1128/jvi.63.7.2987-2994.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand K. H., Long C. W. Fusion of a Rous sarcoma virus transformed human cell line, KC, by RD-114 virus. J Gen Virol. 1973 Dec;21(3):523–532. doi: 10.1099/0022-1317-21-3-523. [DOI] [PubMed] [Google Scholar]
- Tan T. H., Jia R., Roeder R. G. Utilization of signal transduction pathway by the human T-cell leukemia virus type I transcriptional activator tax. J Virol. 1989 Sep;63(9):3761–3768. doi: 10.1128/jvi.63.9.3761-3768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A., Lundin K., Patarroyo M., Asjö B. The leukocyte adhesion glycoprotein CD18 participates in HIV-1-induced syncytia formation in monocytoid and T cells. J Immunol. 1990 Feb 1;144(3):934–937. [PubMed] [Google Scholar]
- Varmus H. Retroviruses. Science. 1988 Jun 10;240(4858):1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- Volkman L. E., Krueger R. G. XC cell cytopathogenicity as an assay for murine myeloma C-type virus. J Natl Cancer Inst. 1973 Oct;51(4):1205–1210. doi: 10.1093/jnci/51.4.1205. [DOI] [PubMed] [Google Scholar]
- Vyth-Dreese F. A., de Vries J. E. Enhanced expression of human T-cell leukemia/lymphoma virus in neoplastic T cells induced to proliferate by phorbol ester and interleukin-2. Int J Cancer. 1983 Jul 15;32(1):53–59. doi: 10.1002/ijc.2910320109. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P., Nagy K., Hoshino H. Envelope properties of human T-cell leukemia viruses. Curr Top Microbiol Immunol. 1985;115:235–246. doi: 10.1007/978-3-642-70113-9_15. [DOI] [PubMed] [Google Scholar]
- Wolfson M., McPhail L. C., Nasrallah V. N., Snyderman R. Phorbol myristate acetate mediates redistribution of protein kinase C in human neutrophils: potential role in the activation of the respiratory burst enzyme. J Immunol. 1985 Sep;135(3):2057–2062. [PubMed] [Google Scholar]
- Yip M. T., Chen I. S. Modes of transformation by the human T-cell leukemia viruses. Mol Biol Med. 1990 Feb;7(1):33–44. [PubMed] [Google Scholar]