Abstract
A cDNA encoding a cytochrome P450 enzyme was isolated from a cDNA library of the corpora allata (CA) from reproductively active Diploptera punctata cockroaches. This P450 from the endocrine glands that produce the insect juvenile hormone (JH) is most closely related to P450 proteins of family 4 and was named CYP4C7. The CYP4C7 gene is expressed selectively in the CA; its message could not be detected in the fat body, corpora cardiaca, or brain, but trace levels of expression were found in the midgut and caeca. The levels of CYP4C7 mRNA in the CA, measured by ribonuclease protection assays, were linked to the activity cycle of the glands. In adult females, CYP4C7 expression increased immediately after the peak of JH synthesis, reaching a maximum on day 7, just before oviposition. mRNA levels then declined after oviposition and during pregnancy. The CYP4C7 protein was produced in Escherichia coli as a C-terminal His-tagged recombinant protein. In a reconstituted system with insect NADPH cytochrome P450 reductase, cytochrome b5, and NADPH, the purified CYP4C7 metabolized (2E,6E)-farnesol to a more polar product that was identified by GC-MS and by NMR as (10E)-12-hydroxyfarnesol. CYP4C7 converted JH III to 12-trans-hydroxy JH III and metabolized other JH-like sesquiterpenoids as well. This ω-hydroxylation of sesquiterpenoids appears to be a metabolic pathway in the corpora allata that may play a role in the suppression of JH biosynthesis at the end of the gonotrophic cycle.
Keywords: corpora allata/CYP4C7/P450 reconstitution/sesquiterpenoid/NMR
Juvenile hormone (JH) plays a central role in insect development, metamorphosis, and reproduction. This sesquiterpenoid epoxide is synthesized in endocrine glands, the corpora allata (CA)(1), and is degraded predominantly by esterases and epoxide hydrolases (2). The rate of JH synthesis by the CA is a major determinant of the titer of JH in the hemolymph (3), and the regulation of JH synthesis is seen as a potential target for insect control. The biosynthesis of JH has been extensively characterized during the reproductive cycle of the cockroach Diploptera punctata, an insect that serves as a convenient model system. In adult females of this insect, the cycle of JH synthesis is regulated by humoral factors and by innervation from neurosecretory cells in the brain (1). Production of JH by the CA increases 10-fold to reach a peak 5 days after adult emergence and mating, and this peak corresponds to the peak of vitellogenesis. Synthesis is then rapidly repressed and remains low from deposition of the eggs into the brood sac through pregnancy (4).
The allatostatins, a family of brain-gut peptides (5–9) are known to inhibit JH synthesis in D. punctata at the end of a gonotrophic cycle, but these peptides are probably not alone responsible for repression of the intrinsic rate of JH synthesis in postvitellogenic insects. The mechanism of stable suppression of JH synthesis at the end of the cycle has received very little attention, in part because of the absence of molecular tools to dissect it. This repression occurs concomitant with changes in cell morphology (10–12) but not cell number (12, 13), and it is thought to involve regulation of enzymes of the biosynthetic pathway. However, the activity of the pathway enzymes studied to date has always been higher than that required for the intrinsic rates of JH synthesis (i.e., they are not rate-limiting): both hydroxymethylglutaryl (HMG)-CoA synthase (14) and HMG-CoA reductase (15) activities peak on day 5 but do not decline until 24 and 48 hr, respectively, following the decline in JH synthesis. The O-methyltransferase activity is highest on day 3 and remains particularly high until day 7 (16), and farnesoic acid-stimulated rates of JH synthesis far exceed intrinsic rates (17), thus showing that the terminal enzyme activities are also in excess of those required to account for JH synthetic rates. These observations, taken together, suggest that other mechanisms may be involved in the intrinsic regulation of JH synthesis during a normal cycle of activity. Furthermore, hemolymph JH esterase levels, induced by JH, increase only after JH synthetic levels have already declined (18), so that JH esterase alone cannot depress JH levels at the end of vitellogenesis. Rather, it appears to be a scavenger enzyme working in conjunction with a decrease in biosynthesis to reduce JH to a very low level.
The importance of cytochrome P450 enzymes in hormone biosynthesis and metabolism (19) and the known role of one such P450 in the epoxidation of methyl farnesoate to insect JH (20, 21) motivated us to search for P450 genes expressed in the CA. In this paper we report the cloning and characterization of a cytochrome P450, CYP4C7, that is strongly expressed in the CA of postvitellogenic insects. The CYP4C7 protein expressed in a heterologous system metabolizes JH III and JH precursors to their 12-trans-hydroxy metabolites. The profile of expression of this gene and the catalytic competence of its product suggest that it encodes an enzyme involved in regulating levels of JH synthesis by metabolizing JH precursors and JH itself within the CA during specific developmental stages.
MATERIALS AND METHODS
Chemicals and Insects.
The cis- and trans-12-hydroxy JH III standards were kindly supplied by F. Couillaud (Centre National de la Recherche Scientifique) and G. D. Prestwich (University of Utah), and other terpenoids were as described in Andersen et al. (22). D. punctata were reared as described (23).
Isolation and Cloning of CYP4C7.
The degenerate PCR primer, GA(C/T) ACI TT(C/T) ATG TT(C/T) GA(A/G) GG(A/G/C/T) CA(C/T) GA(C/T) AC and the reverse degenerate primer GC(A/G) AT(C/T) TT(C/T) TG(A/G/C/T) CC(A/G/T) AT(A/G) CA(A/G) TT were used to amplify a 452-bp DNA fragment from reverse-transcribed poly(A)+ RNA isolated from 25 pairs of CA from 5-day-old mated females. This amplicon was cloned into the pCR II vector (Invitrogen) and used to isolate an almost-full-length clone from a CA cDNA library. The library was constructed from 240 pairs of CA from 5-day-old mated females by using the Zap-cDNA synthesis kit (Stratagene).
RNase Protection Assays.
A riboprobe template for CYP4C7 was prepared by digesting the pBluescript plasmid with ScaI. Antisense 32P-labeled RNA fragments were synthesized by runoff transcription from the T7 promoter by using the MAXIscript in vitro transcription kit (Ambion), which generated a probe of 175 bp and a protected fragment of 145 bp corresponding to the 3′ region of the CYP4C7 message. RNA protection assays were performed by using the Direct Protect lysate ribonuclease protection assay kit from Ambion. The CA were collected in 50 μl of lysate buffer and stored at −80°C until they were assayed. The products of the assay, protected RNA–RNA hybrids, were separated on a denaturing 6% acrylamide gel, and either visualized by autoradiography with the amount of signal quantified by an LKB densitometer or directly quantified by using a PhosphorImager (Molecular Dynamics).
Construction of Expression Vector.
The coding region of CYP4C7 was cloned into the expression plasmid pSE380 following modification by PCR mutagenesis. The AT content of the 5′ region was increased with PCR mutagenesis by using the primer C ATG GCT GTT TTA TTA CTT ACT TCT CTT GCT ATA GTC. Further PCR mutagenesis with the primer GGG GTA CCT CAG TGA TGG TGG TGT GTT CTA GCA GTG AT was used to add four histidine codons to the 3′ end of the coding region to allow enzyme purification by nickel-chelate chromatography.
Purification of CYP4C7 from Escherichia coli.
Flasks containing 500 ml of Terrific Broth (with ampicillin at 50 μg/ml) were inoculated with 500 μl of an overnight culture of E. coli transformed with the CYP4C7 expression plasmid. The flasks were shaken at 250 rpm and 37°C until the OD550 of the culture was ≈1.0. Isopropyl β-d-thiogalactoside (IPTG) was added to 1 mM and δ-aminolevulinic acid was added to 0.5 mM, and the flasks were shaken at 27°C and 125 rpm for 48 hr. The cells were harvested at 3,500 × g, washed with Tris⋅HCl buffer (0.1 M, pH 7.7), and pelleted a second time. The cell pellet was resuspended in 40 ml of Tris⋅HCl buffer (0.1 M, pH 7.7) and 10% glycerol, then sonicated. Phenylmethylsulfonyl fluoride (PMSF) was added to achieve a 0.2 mM concentration immediately before each sonication. The membrane fraction was pelleted by centrifugation at 100,000 × g for 30 min and resuspended in 50 ml of Tris⋅HCl buffer (0.1 M, pH 7.7) and 20% glycerol. CYP4C7 was solubilized from the membranes by adding Emulgen 911 to achieve a 1% concentration, and the product was stirred at 4°C for 4 hr. Solubilized proteins were recovered in the supernatant fraction after a further centrifugation at 100,000 × g for 30 min. The supernatant was loaded onto a nickel affinity column equilibrated with Tris⋅HCl buffer (20 mM, pH 7.7), 20% glycerol, 0.5 M NaCl, and 5 mM imidazole (equilibration buffer). The column was washed with 50 ml of equilibration buffer with Emulgen 911 to 0.1%, then with equilibration buffer with 0.3% sodium cholate. CYP4C7 was eluted with equilibration buffer with 200 mM imidazole. The P450-containing fractions were pooled and dialyzed against three changes of Tris⋅HCl buffer (100 mM, pH 7.7), 20% glycerol, and 0.1 mM EDTA.
Spectral Studies.
Cytochrome P450 was quantified by measurement of the dithionite-reduced vs. reduced-CO bound-difference spectrum by the method of Omura and Sato (24). Type I difference spectra of ligands (added in ethanol) were obtained from an 800-μl suspension of the membrane fraction of (E. coli + plasmid) in 100 mM Mops containing 0.825 μM cytochrome P450.
Enzyme Assay.
Cytochrome P450 enzyme assays were performed in 0.1 M Tris⋅HCl (pH 7.7) containing 20 μM NADPH, an NADPH-regenerating system (1 mM glucose-6-phosphate, 2 units/ml of glucose-6-phosphate dehydrogenase), 0.2 μM CYP4C7, 0.5 μM recombinant housefly NADPH-dependent cytochrome P450 reductase (ref. 25, and M.B.M., A. Ariño, V. M. Guzov, and R.F., unpublished results), 1 μM cytochrome b5 (26), and 100 μM substrate. The enzymes were reconstituted by adding, in the following order, 0.1 mg/ml dilauroylphosphatidylcholine, 0.015% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate), CYP4C7, cytochrome b5, and house fly P450 reductase. Buffer was then added to increase the volume to 40 μl, and the mix was incubated on ice for 10 min. Reactions were started by adding 1 vol of protein mix to 9 vol of substrate solution and proceeded for 15–20 min at 30°C. Products were extracted with 2 ml of peroxide-free ethyl ether, methylated with diazomethane when appropriate, dried under a stream of N2, resuspended in hexane, and analyzed by GC with a flame ionization detector.
NMR Spectroscopy of the Farnesol Metabolite.
A large-scale preparation of the principal farnesol metabolite was partially purified by reversed-phase HPLC, and the oil (1–2 mg) was dissolved in tetrahydrofuran (THF)-d8 for preliminary evaluation by 500-MHz 1H NMR spectroscopy. The oil consisted of a major component (≈60%) and one or more minor components (≈40%) that did not appear to be farnesol-derived. Following removal of the THF, the oil was triturated with benzene-d6, and undissolved material was removed by filtration. NMR analysis confirmed that the minor components had been substantially eliminated. As THF-d8 appeared to be a superior solvent for NMR analyses, the benzene was removed, and the oil was redissolved in THF-d8. The spectra were obtained in THF-d8 solutions on a Bruker (Billerica, MA) WM 500 spectrometer. Chemical shifts are reported in δ. The reference signal for the proton spectra (1.73 ppm) was caused by residual protons at C3 and C4 in the THF-d8. The reference signal for the carbon spectra (25.37 ppm) was the result of the signal from C3 and C4 in the THF-d8. 1H NMR δ 5.36 (1, t, H at C10), 5.35 (1, t, H at C2), 5.17 (1, t, H at C2), 4.02 (2, dd, H at C1), 3.84 (2, d, H at C12), 3.52 (1, t, OH at C12), 3.30 (1, t, OH at C1), 2.12 (4, dt, H at C5 and C9), 2.01 (4, t, H at C4 and C8), 1.63 (3, s, CH3), 1.62 (3, s, CH3), 1.61 (3, s, CH3). 13C NMR δ 136.48 (C3), 136.33 (C11), 135.16 (C7), 126.40, 124.80, 124.18 (C2, C6, C10), 68.15 (C12), 59.05 (C1), 38.08, 38.07 (C4, C8), 27.03, 26.98 (C5, C9), 15.85, 15.78, 13.35 (CH3).
Microchemical Derivatizations of the JH III Metabolite.
Derivatizations were a modification of the procedures of Bergot et al. (27, 28). Fifty microliters of a solution of 1 μl of 60% perchloric acid in 1 ml of methyl-d3 alcohol-d (CD3OD) (99.5 atom %) was added to ≈ 1 μg of CYP4C7 product. After 30 min at room temperature, 0.5 ml of a 2% NaCl solution was added, and the mixture was extracted three times with 100 μl of ethyl acetate. The combined organics were evaporated under N2 until dry. The product was dissolved in 10 μl of ethyl acetate, and 2 μl of this solution was injected into the GC-MS. For trimethylsilylation, 50 μl of bis (trimethylsilyl)trifluoracetamide (BSTFA) was added to ≈1 μg of CYP4C7 product and incubated at 55°C for 1 hr. The reaction mixture was evaporated to dryness under N2. Fifty microliters of ethyl acetate was added and evaporated. The product was dissolved in 20 μl of ethyl acetate, and 2 μl of this solution was injected into the GC-MS.
GC-MS.
Metabolites were analyzed with a Hewlett-Packard 5890 GC coupled to an HP 5970 mass selective detector at 70-eV ionization, with total ion detection from 30 to 350 atomic mass units. The GC column was a J & W Scientific (Folsom, CA) DB-5ms, (5%-phenyl)-methylpolysiloxane column, 25 m × 0.32 mm i.d. × 0.52-μm film. The temperature profile was 100°C for 2 min, then 10°C increase per min to 250°C and held for 3 min. The injector temperature was 250°C and the detector was set at 280°C, with helium carrier gas at a linear velocity of 28.7 cm/sec.
RESULTS
Isolation, Cloning, and Sequencing of the CYP4C7 Gene.
A reverse transcription–PCR (RT-PCR) approach (29) for amplifying and cloning P450 fragments was used on mRNA from the CA of reproductively active female D. punctata cockroaches. This approach used degenerate primers for the consensus sequence of the helix I and of the heme-binding regions of previously isolated CYP4 P450s. It gave a 452-bp product that when cloned and sequenced revealed high sequence similarity to known P450 proteins. A specific forward primer for this P450 fragment (GCA TCA GGA GTG AGC TG), when used with the degenerate reverse primer, generated an RT-PCR product from CA mRNA but not from abdominal-fat-body mRNA. In positive-control experiments, the combination of degenerate primers produced a signal from abdominal-fat-body mRNA. When cloned and sequenced, the fat-body RT-PCR product gave three different P450 fragments (CYP4C4, CYP4C5, and CYP4C6).
The cDNA for the allatal P450 from a CA cDNA library was then isolated. We obtained a clone that has an ORF of 497 amino acids, is most related to P450s of the CYP4 family, and was named CYP4C7 (44.3% identity to Blaberus discoidalis CYP4C1, 34.9% identity to Drosophila melanogaster CYP4D1, and 36.7% identity to Manduca sexta CYP4M2). The reading frame of the cDNA lacked a start codon, and the alignment with other CYP4 proteins (Fig. 1) showed that 4–10 N-terminal residues, at most, were missing from the hydrophobic anchor typical of microsomal P450 proteins (31). In addition to the highly conserved sequence surrounding the cysteine ligand to the heme (PFSAGPRNCIGQKFA), there is also a highly conserved sequence (IXEEVDTFMLXGHDT) in the putative I helix, preceding the threonine residue at the proposed oxygen-binding pocket.
Figure 1.
Alignment of the deduced amino acid sequence of the allatal CYP4C7 and fat body CYP4C4, 5, and 6 from D. punctata, with other insect CYP4 proteins (CYP4C1 from B. discoidalis, CYP4D1 from D. melanogaster, and CYP4M2 from M. sexta). The alignment was produced with clustal x. The positions of the primers used for initial RT-PCR are shown as arrows in the conserved I helix region and Cys heme-ligand region. Asterisks mark residues shared by all four or seven sequences.
CYP4C7 Expression Is Linked to the Activity Cycle of the CA.
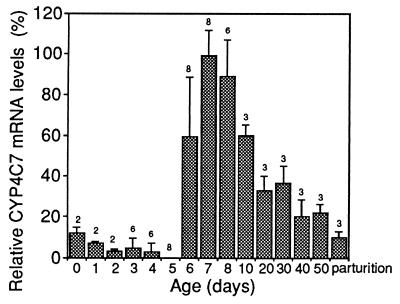
We tested whether this P450 was linked to the activity cycle of the CA in adult females (low JH synthesis on day 0, increasing to a peak on day 5 after adult emergence, followed by a rapid decline, then low activity again during pregnancy). Thus we followed expression of the CYP4C7 gene by ribonuclease protection assays on RNA from the CA, to ensure absolute specificity as well as high sensitivity. The CYP4C7 message is predominantly expressed when the activity of the CA is shut off, starting on day 6 after the peak of JH synthesis and peaking on day 7–8, just before oviposition. The message then gradually declines after oviposition and during pregnancy (Fig. 2). Significant levels of message are found shortly after adult emergence, but the lowest levels are found during vitellogenesis, with undetectable levels of CYP4C7 on day 5. Males and virgin females had 10% and 13%, respectively, of the level of message observed in 7-day-old mated females.
Figure 2.
CYP4C7 mRNA levels in the CA of adult mated females. The values are means ± SD of n pairs of CA as shown above bars. The levels were measured by RNase protection assays and expressed as a percentage of the value of day-7 CA taken as arbitrary standard.
The RNase protection method confirmed the initial RT-PCR result that CYP4C7 mRNA was not detectable in the fat body. This technique showed no detectable expression in the head or abdominal fat body of 1-, 5-, or 9-day-old insects or the corpora cardiaca or brain of 5-day-old insects. There was also no detectable expression in the postvitellogenic ovary, the prothoracic gland from late final-instar larvae, or the tissue remnants of that gland in newly emerged females. Trace levels of expression were observed in the midgut of newly emerged, 4-day-old and 8-day-old mated females and in the gastric caeca from 4-day-old insects. Relative to the fresh weight of the tissues, the mRNA levels in the caeca and midgut were 0.20% and 0.10–0.26%, respectively, of the level in a single CA from 7-day-old females. Thus, expression of CYP4C7 was 500-fold higher in the CA than in the midgut or gastric caeca.
Heterologous Expression of the CYP4C7 Protein.
The cell lysate of E. coli cells transformed with the CYP4C7 expression vector contained cytochrome P450 as detected by the characteristic reduced CO/reduced difference spectrum with a peak at 448 nm. The expression construct encoded a 4-His tag that allowed purification of the enzyme on a nickel affinity column. The eluate from the affinity column contained a major protein band of 50–60 kDa after analysis on SDS/PAGE (not shown), with spectra of oxidized, reduced, and CO-bound reduced purified protein typical of P450 proteins (not shown). The level of cytochrome P450 expression was about 140 nmol/liter of culture.
Membrane-bound CYP4C7 gave type I binding spectrum with (2E,6E)-methyl farnesoate and with JH III (spectral binding constant Ks of 0.9 and 2.4 μM, respectively), whereas the affinity for lauric acid, which is not a substrate for CYP4C7 (see below) was lower (74 μM). The purified recombinant CYP4C7 was reconstituted with the purified recombinant housefly P450 redox partners NADPH cytochrome P450 reductase and cytochrome b5; this functional enzyme system was used to study the metabolism of potential substrates.
Metabolism of Farnesol by CYP4C7 and Identification of (10E)-12-Hydroxyfarnesol.
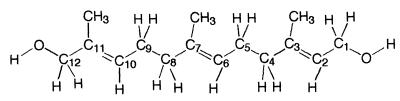
(2E,6E)-Farnesol, a precursor of JH III, was metabolized to two more polar compounds. When analyzed by GC-MS, the principal metabolite had diminished abundance of the m/z 69 ion and increased abundance of the m/z 31 ion of farnesol. It was converted by treatment with BSTFA to a compound bearing two trimethylsilyl groups, suggesting the presence of an additional hydroxyl group. The observed 1H NMR spectrum of the principal metabolite was consistent with that of a hydroxylated farnesol. The appearance of three methyl singlets (1.61, 1.62, and 1.63 ppm) and a doublet (coupled to a hydroxyl proton) at 3.84 ppm suggested that hydroxylation had occurred on C12. Mass-spectral fragmentation patterns suggested that the hydroxymethyl substituent resided on C11 (farnesol numbering, see Fig. 3). The structure of this metabolite was unambiguously determined to be a single stereoisomer of 12-hydroxyfarnesol by a combination of heteronuclear multiple-quantum coherence and heteronuclear multiple-bond coherence experiments.
Figure 3.
Structure of the 12-hydroxyfarnesol produced from farnesol by CYP4C7.
Stereochemistry at the C2⩵C3 and C6⩵C7 double bonds was assumed to be E in the metabolite, in keeping with the stereochemistry of the starting material. Support for this assumption comes from the similarity of the NMR spectra of the metabolite to the spectra of E,E-farnesol. Because only one stereoisomer about the C10⩵C11 double bond was available, nuclear Overhauser effect experiments were not a reliable means for assigning this stereochemistry (31). However, Vogeli and von Philipsborn (32) were able to assign alkene stereochemistry on the basis of chemical shifts and vicinal C–H spin-coupling constants. In the model compound Z-2-methyl-2-buten-1-ol, the C1-carbon chemical shift is 60.5 ppm and the C2-methyl chemical shift is 21.3 ppm, whereas for E-2-methyl-2-buten-1-ol these values are 68.3 ppm and 13.3 ppm, respectively. For the 12-hydroxyfarnesol metabolite, the C12 chemical shift is 68.2 ppm and the C11-methyl chemical shift is 13.4 ppm, consistent with 10E stereochemistry. Further support for this assignment comes from vicinal C–H spin-coupling constants. For Z-2-methyl-2-buten-1-ol, the vicinal C–H spin-coupling constant 3JC1-H3 = 9.3 Hz, whereas for E-2-methyl-2-buten-1-ol 3JC1-H3 = 7.6 Hz. For the 12-hydroxyfarnesol metabolite the vicinal C–H spin-coupling constant 3JC2-H10 = 7.4 Hz. These data all concur to identify the principal farnesol metabolite of CYP4C7 as (10E)-12-hydroxyfarnesol.
Metabolism of JH III by CYP4C7 and Identification of (10E)-12-Hydroxy-JH III.
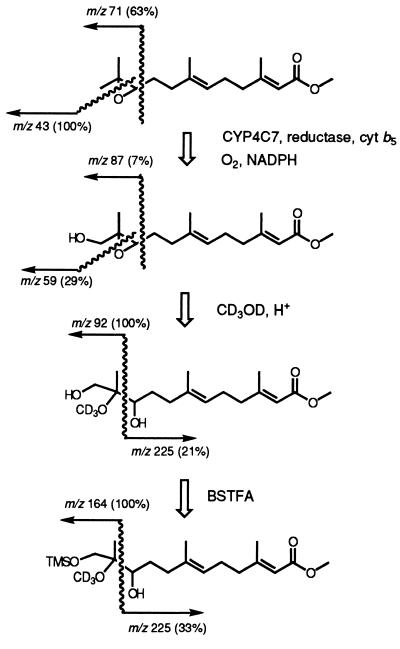
JH III was metabolized to two more polar products; the major one was identified by a combination of microderivatization and GC-MS analysis (Fig. 4), which indicated the presence of a terminal hydroxyl group as for the farnesol metabolite. JH III has a complex electron-impact fragmentation pattern (33), and under the conditions we used had a retention time of 16.3 min. Major fragment ions were at m/z 43(100%), 81(84%), and 41(70%). Under the same chromatographic conditions the major JH III metabolite had a retention time of 19.02 min and major ions at m/z 55(100%), 93(92%), and 81(90%). Its deuteroxyhydrin derivative had a retention time of 22.53 min and gave major ions at m/z 92(100%), 75(63%), and 193(48%). The trimethylsilyl derivative of the CYP4C7 product had a retention time of 20.02 min, a strong m/z 73(100%) ion indicative of a single trimethylsilyl product, and other major ions at m/z 93(70%) and 55(53%). BSTFA did not react under our conditions to silanize the hydroxyl on C10 of the JH III deuteroxyhydrin derivative. Trimethylsilylation of the deuteroxyhydrin derivative gave a product with a retention time of 22.51 min with major ions at m/z 164(100%), 73(71%), and 75(57%). These results (Fig. 4) imply that the major JH III metabolite is a 12- or 12′-hydroxy derivative of JH III. To determine which geometric isomer of 12-hydroxy JH III was produced, authentic (10Z)-and (10E)-12-hydroxy JH III were separated by GC and compared with the major product of CYP4C7 metabolism. The biosynthesized 12-hydroxy JH III had the same retention time as the synthetic (10E)-12-hydroxy JH III standard. The minor metabolite of JH III did not correspond to the 10Z isomer, and its structure has not been elucidated.
Figure 4.
Structure determination of the (10E)-12-hydroxy-JH III, showing the mass fragmentation scheme of JH III, its enzymatic product and derivatives, and abundances of characteristic ions. TMS, trimethylsilyl
Metabolism of Sesquiterpenoids by CYP4C7.
Table 1 shows that CYP4C7 is active toward sesquiterpenoid compounds. The selectivity toward JH III and its precursors is marked. The enzyme is more active toward 10,11-unsaturated compounds than toward the 10,11-epoxides, but the nature of the functionality at the opposite end of the molecule (alcohol, aldehyde, carboxylic acid, methyl ester, or methyl ether) appears of less importance. Monoterpenes and diterpenes are not metabolized, nor is the saturated analog of farnesol (2,6,10-trimethyldodecanol). This selectivity suggests that the enzyme is not involved in biosynthesis, but rather is involved in metabolizing JH precursors (starting with farnesol) to more polar compounds, presumably to facilitate their further catabolism and disposition. CYP4C7 had no activity toward either fatty acids (laurate or palmitate), which are typical substrates of P450s of the CYP4 family in mammals, or cyclodiene insecticides (aldrin or heptachlor).
Table 1.
Substrate selectivity of reconstituted CYP4C7
| Substrate | pmol product/pmol P450⋅min−1
|
|
|---|---|---|
| Major metabolite | Minor metabolite | |
| (2E,6E)-Farnesol | 4.12 ± 0.50 | 0.92 ± 0.12 |
| (2E,6E)-Farnesal | 2.37 ± 0.90* | |
| (2E,6E)-Farnesoic acid | 2.62 ± 0.20 | ND |
| (2E,6E)-Methyl farnesoate | 2.26 ± 0.17 | 0.71 ± 0.01 |
| 10,11-Epoxy-(2E,6E)-farnesoic acid | ND | |
| JH III | 0.83 ± 0.27 | 0.47 ± 0.04 |
| JH II | 0.87 ± 0.17 | 0.19 ± 0.07 |
| JH I | 0.41 ± 0.03 | 0.17 ± 0.02 |
| 2,6,10-Trimethyldodecanol | ND | |
| Geraniol | ND | |
| Geranyl geraniol | ND | |
| Farnesyl methyl ether | 2.10 ± 0.30 | 0.65 ± 0.10 |
| Geranyl methyl ether | 0.17 ± 0.02 | 0.16 ± 0.04 |
Purified CYP4C7 were reconstituted with house fly P450 reductase and cytochrome b5, and an NADPH-regenerating system. Nominal substrate concentration was 100 μM. Values are means ± SD of triplicate assays. ND, no product detected.
Substrate disappearance, product not identified.
Effect of Topically Applied JH III at the End of the Gonotrophic Cycle.
The effect of JH III at the end of the cycle was studied by topical application on day 6 or by repeated applications on days 6, 7, and 8. The high doses and repeated applications were necessary in light of the notoriously rapid in vivo degradation of the natural JH III. The results (Table 2) show that JH had two deleterious effects: inhibition of oviposition and abortion of oviposited eggs. There was no resorption of oocytes in nonovulated females, as the oocytes were already chorionated at the time of JH treatment; in these insects, the mature eggs shrunk gradually, releasing the contents and leaving the chorion remains as pigmented spherical bodies. There was no vitellogenesis in the penultimate oocytes in nonovulated and aborted females until 5 wk after JH treatment (at which time the experiment was terminated). These results strongly suggest that JH titers need to decrease rapidly when vitellogenesis and yolk uptake by the oocytes is complete for successful reproduction to occur.
Table 2.
Effect of topical application of JH III after completion of vitellogenesis
| Exp. | Inhibition of ovulation, % | Abortion, % |
|---|---|---|
| 1 (single application) | 52.5 ± 2.5 | 41.7 ± 12.7 |
| 2 (repeated applications) | 66.7 ± 3.3 | 72.2 ± 14.7 |
JH III was applied topically in acetone after completion of vitellogenesis as a single dose (100 μg) on day 6 (Exp. 1), or in repeated doses (100 μg each) on days 6, 7, and 8 (Exp. 2). Results are means ± SE of 4 or 3 replicates of 10 females. All control insects treated with acetone along ovulated, and only 7.7% aborted (n > 30). Abortion is shown as percent of females that had ovulated. Abortion occurs within 2 wk after ovulation.
DISCUSSION
Cloning and Characterization of CYP4C7.
Cloning of cytochrome P450 genes by the RT-PCR method has been highly successful in documenting P450 diversity in plants and insects (29). Here it has allowed cloning of a P450 gene that is selectively expressed in the CA. These small endocrine glands are not easily studied by classical biochemical techniques, and reliance on radioenzymological assays has been absolute (34). Heterologous expression of a CA enzyme thus provides a new tool in the study of insect endocrine biochemistry. CYP4C7 contains a region of high sequence similarity to other members of the CYP4 family (Fig. 1), and several CYP4 enzymes of vertebrates have been implicated in ω-hydroxylation of fatty acids as well as leukotriene hydroxylation (30). CYP4C1 of another cockroach, Blaberus discoidalis, is a P450 expressed in the fat body that is thought to be involved in lipid mobilization under the control of the hypertrehalosemic hormone (35). Because high CYP4C7 mRNA levels are found in the CA at the end of vitellogenesis, a time of rapid cell shrinking (but not cell death) in the glands, it was possible that the enzyme was involved in recycling membrane lipids (fatty acids) within the CA. However, we discounted this possibility for several reasons. First, the purified, reconstituted enzyme does not metabolize the fatty acids laurate or palmitate. Second, CYP4C7 is expressed selectively in the CA, with only very limited expression in the midgut and no detectable expression in the postvitellogenic ovary or late-larval prothoracic glands, two tissues where massive cellular degradation is also taking place. Third, CYP4C7 mRNA levels increase in the CA of ovariectomized insects, yet these glands do not shrink in size and are competent to produce high levels of JH (T.D.S., G.C.U., and R.F., unpublished results). Thus the function of CYP4C7 in the CA is unlikely to be that of a housekeeping enzyme, but is more likely to be directly involved in the JH biosynthetic pathway, as reflected by its substrate selectivity toward sesquiterpenoids.
Function of the Enzyme.
There is evidence in other invertebrate species that JH-related compounds may act as hormones or prohormones, for instance the production of farnesoic acid and methyl farnesoate in crustaceans (36). In insects, the higher homologs of JH III and their respective JH acids are produced by the CA of Lepidoptera (34); other JH-related molecules have been reported as products of the CA as well. These include JH III diol in locusts (37), a very minor product of the CA in this species (38), JH III bisepoxide in higher Diptera (39, 40), and what appears to be a JH acid glucuronide in Manduca sexta (41). The JH III bisepoxide is believed to be an authentic JH of higher Diptera, but the role of the other products is less clear. Of direct interest to our present study, a (10Z)-12 hydroxy metabolite of JH III (12′-OH JH III) has recently been identified in in vitro incubations of the CA in locusts (42), and several other metabolites have been reported as well, including 8′-OH JH III (38, 42). In the locust, synthesis of the hydroxy-JH metabolites is not increased by farnesol or farnesoic acid, whereas synthesis of JH is stimulated by these exogenous precursors (38). This suggests either a different level of saturation of the hydroxylase(s) or a physical compartmentation of the pathways of JH III synthesis from endogenous and exogenous substrates. Interestingly, locust CA produced the cis-hydroxy JH III, whereas the cockroach P450 enzyme makes the trans-hydroxy JH III from JH III and trans-hydroxyfarnesol from farnesol. As yet, we do not know whether CYP4C7 is acting primarily to catabolize JH-related compounds or whether any of the metabolites are capable of functioning as hormones or prohormones. The 12′-OH JH III produced by locust CA has been reported to have a very slight activity in the Tenebrio test for JH activity, about 1/100 that of JH itself (42). This metabolite has retained both the epoxide and the methyl ester functionalities that are essential for biological activity.
We have not yet identified any new product of the CA, but we have characterized an enzyme that is probably homologous to the enzyme producing 12′-OH JH III in locusts. CYP4C7 gene expression within the CA of mated female D. punctata is inversely correlated to JH synthesis from the gland. The timing of CYP4C7 gene expression and its catalytic activity suggests that this enzyme is involved in the catabolism of sesquiterpenoids within the CA during the developmental stages, when an intrinsic repression of JH synthesis is observed. The effect of JH or its agonists on adult insects has usually been measured in terms of hormone replacement therapy, showing that JH can restore egg growth in allatectomized insects. We show that in this viviparous cockroach, JH excess at the end of the cycle causes a distinct pathological syndrome, inhibition of ovulation and abortion of early embryos. This highlights the need for a rapid decrease in JH titer once eggs are ready to be ovulated. Excess JH (analog) in adult cockroaches is known to have adverse effects on reproduction (43, 44). CYP4C7 expression is high in postvitellogenic insects, when a reduction in JH titer is critical for allowing oviposition and preventing abortion, as suggested by our data on topical application of JH III (Table 2).
We still need to identify which if any 12-hydroxy sesquiterpenoids are produced by the CA at the end of the gonotrophic cycle to determine which metabolite(s) is the preferred substrate of CYP4C7 in vivo. Drosophila Kc cells (which lack squalene synthase) as well as rats (treated with farnesol or with a squalene synthase inhibitor) convert farnesol to dicarboxylic acids (45, 46) in a pathway that is initiated by ω-hydroxylation of farnesol. By analogy to these known catabolic pathways, it is possible that CYP4C7 is only the first enzyme in the catabolism of allatal sesquiterpenoids. The CYP4C7 gene can now be used as a molecular probe to study the early postvitellogenic events in D. punctata, when signals from the ovary and the brain converge on the CA to shut down JH synthesis.
Acknowledgments
We thank Drs. F. Couillaud and G. D. Prestwich for authentic samples of 12-hydroxy JH III. This work was supported by National Institutes of Health Grants DK34549 to R.F. and by Center Grant ES06694 which supported work in the synthetic core, Southwest Environmental Health Sciences Center.
ABBREVIATIONS
- BSTFA
bis[trimethyl-silyl]trifluoracetamide
- CA
corpora allata
- JH
juvenile hormone
- RT
reverse transcription
- THF
tetrahydrofuran
Footnotes
References
- 1. Feyereisen R. In: Comprehensive Insect Physiology, Biochemistry and Pharmacology. Kerkut G A, Gilbert L I, editors. Vol. 7. Oxford: Pergamon; 1985. pp. 391–429. [Google Scholar]
- 2.Hammock B D. In: Comprehensive Insect Physiology, Biochemistry and Pharmacology. Kerkut G A, Gilbert L I, editors. Vol. 7. Oxford: Pergamon; 1985. pp. 431–472. [Google Scholar]
- 3.De Kort C A D, Granger N A. Annu Rev Entomol. 1981;26:1–28. [Google Scholar]
- 4.Tobe S S, Ruegg R P, Stay B A, Baker F C, Miller C A, Schooley D A. Experientia. 1985;41:1028–1034. doi: 10.1007/BF01952127. [DOI] [PubMed] [Google Scholar]
- 5.Woodhead A P, Stay B, Seidel S L, Khan M A, Tobe S S. Proc Natl Acad Sci USA. 1989;86:5997–6001. doi: 10.1073/pnas.86.15.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pratt G E, Farnsworth D E, Siegel N R, Fok K F, Feyereisen R. Biochem Biophys Res Commun. 1989;163:1243–1247. doi: 10.1016/0006-291x(89)91111-x. [DOI] [PubMed] [Google Scholar]
- 7.Pratt G E, Farnsworth D E, Fok K F, Siegel N R, McCormack A L, Shabanowitz J, Hunt D F, Feyereisen R. Proc Natl Acad Sci USA. 1991;88:2412–2416. doi: 10.1073/pnas.88.6.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodhead A P, Khan M A, Stay B, Tobe S S. Insect Biochem Mol Biol. 1994;24:257–263. doi: 10.1016/0965-1748(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 9.Reichwald K, Unnithan G C, Davis N T, Agricola H, Feyereisen R. Proc Natl Acad Sci USA. 1994;91:11894–11898. doi: 10.1073/pnas.91.25.11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelmann F. Biol Bull. 1959;116:406–419. [Google Scholar]
- 11.Szibbo C M, Tobe S S. J Insect Physiol. 1981;27:655–665. [Google Scholar]
- 12.Chiang A S, Tsai W H, Holbrook G L, Schal C. Arch Insect Biochem Physiol. 1996;32:299–313. [Google Scholar]
- 13.Unnithan, G. C., Sutherland, T. D., Cromey, D. & Feyereisen, R. (1998) J. Insect Physiol., in press. [DOI] [PubMed]
- 14.Couillaud F, Feyereisen R. Insect Biochem. 1991;21:131–135. [Google Scholar]
- 15.Feyereisen R, Farnsworth D E. Mol Cell Endocrinol. 1987;53:227–238. doi: 10.1016/0303-7207(87)90178-x. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Ding Q, Yagi K J, Tobe S S. J Insect Physiol. 1994;40:217–223. [Google Scholar]
- 17.Feyereisen R, Friedel T, Tobe S S. Insect Biochem. 1981;11:401–409. [Google Scholar]
- 18.Rotin D, Feyereisen R, Koener J, Tobe S S. Insect Biochem. 1982;12:263–268. [Google Scholar]
- 19.Feyereisen R. Annu Rev Entomol. 1999;44:507–533. doi: 10.1146/annurev.ento.44.1.507. [DOI] [PubMed] [Google Scholar]
- 20.Hammock B D. Life Sci. 1975;17:323–328. doi: 10.1016/0024-3205(75)90479-8. [DOI] [PubMed] [Google Scholar]
- 21.Feyereisen R, Pratt G E, Hamnett A F. Eur J Biochem. 1981;118:231–238. doi: 10.1111/j.1432-1033.1981.tb06391.x. [DOI] [PubMed] [Google Scholar]
- 22.Andersen J F, Walding J K, Evans P H, Bowers W S, Feyereisen R. Chem Res Toxicol. 1997;10:156–164. doi: 10.1021/tx9601162. [DOI] [PubMed] [Google Scholar]
- 23.Meller V H, Aucoin R R, Tobe S S, Feyereisen R. Mol Cell Endocrinol. 1985;43:155–163. doi: 10.1016/0303-7207(85)90079-6. [DOI] [PubMed] [Google Scholar]
- 24.Omura T, Sato R. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- 25.Andersen J F, Utermohlen J G, Feyereisen R. Biochemistry. 1994;33:2171–2177. doi: 10.1021/bi00174a025. [DOI] [PubMed] [Google Scholar]
- 26.Guzov V, Houston H, Murataliev M B, Walker F A, Feyereisen R. J Biol Chem. 1996;271:26637–26645. doi: 10.1074/jbc.271.43.26637. [DOI] [PubMed] [Google Scholar]
- 27.Bergot B J, Jamieson G C, Ratcliff M A, Schooley D A. Science. 1980;210:336–338. doi: 10.1126/science.210.4467.336. [DOI] [PubMed] [Google Scholar]
- 28.Bergot B J, Ratcliff M A, Schooley D A. J Chromatogr. 1981;204:231–244. [Google Scholar]
- 29.Snyder M J, Scott J A, Andersen J F, Feyereisen R. Methods Enzymol. 1996;272:304–312. doi: 10.1016/s0076-6879(96)72036-0. [DOI] [PubMed] [Google Scholar]
- 30.Omura T. In: Cytochrome P450. Schenkman G B, Greim H, editors. Berlin: Springer; 1993. pp. pp.61–69. [Google Scholar]
- 31.Derome A E. Modern NMR Techniques for Chemistry Research. Oxford: Pergamon; 1987. pp. pp.121–122. [Google Scholar]
- 32.Vogeli U, von Philipsborn W. Organic Magnetic Resonance. 1975;7:617–627. [Google Scholar]
- 33.Liedtke R J, Djerassi C. J Org Chem. 1972;37:2111–2119. doi: 10.1021/jo00978a012. [DOI] [PubMed] [Google Scholar]
- 34.Schooley D A, Baker F C. In: Comprehensive Insect Physiology, Biochemistry and Pharmacology. Kerkut G A, Gilbert L I, editors. Vol. 7. Oxford: Pergamon; 1985. pp. 363–389. [Google Scholar]
- 35.Keeley L L, Bradfield J Y, Sowa S M, Lee Y H, Lu K H. In: Perspectives in Comparative Endocrinology. Davey K G, editor. Ottawa: Natural Sciences and Engineering Research Council; 1994. pp. 475–85. [Google Scholar]
- 36.Cusson M, Yagi K J, Ding Q, Duve H, Thorpe A, McNeil J N, Tobe S S. Insect Biochem. 1991;21:1–6. [Google Scholar]
- 37.Gadot M, Goldman A, Cojocaru M, Applebaum S W. Mol Cell Endocrinol. 1987;49:99–108. doi: 10.1016/0303-7207(87)90203-6. [DOI] [PubMed] [Google Scholar]
- 38.Darrouzet E, Rossignol F, Couillaud F. J Insect Physiol. 1998;44:103–111. doi: 10.1016/s0022-1910(97)00100-5. [DOI] [PubMed] [Google Scholar]
- 39.Richard D S, Applebaum S W, Sliter T J, Baker F C, Schooley D A, Reuter C C, Henrich V C, Gilbert L I. Proc Natl Acad Sci USA. 1989;86:1421–1425. doi: 10.1073/pnas.86.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herlt, A. J., Rickards, R. W., Thomas, R. D. & East, P. D. (1993) J. Chem. Soc. Chem. Commun. 1497–1498.
- 41.Granger N A, Janzen W P, Ebersol R. Insect Biochem Mol Biol. 1995;25:427–439. [Google Scholar]
- 42.Darrouzet E, Mauchamp B, Prestwich G D, Kerhoas L, Ujvary I, Couillaud F. Biochem Biophys Res Commun. 1997;240:752–758. doi: 10.1006/bbrc.1997.7739. [DOI] [PubMed] [Google Scholar]
- 43.Staal G B, Henrick C A, Grant D L, Moss D W, Johnston M C, Rudolph R R, Donahue W A. Am Chem Soc Symp Ser. 1985;276:201–218. [Google Scholar]
- 44.Das Y T, Gupta A P. Experientia. 1977;33:968–970. doi: 10.1007/BF01951310. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Pacanowska D, Arison B, Havel C M, Watson J A. J Biol Chem. 1988;263:1301–1306. [PubMed] [Google Scholar]
- 46.Bostedor R G, Karkas J D, Arison B H, Bansal V S, Vaidya S, Germershausen J I, Kurtz M M, Bergstrom J D. J Biol Chem. 1997;272:9197–9203. doi: 10.1074/jbc.272.14.9197. [DOI] [PubMed] [Google Scholar]