Abstract
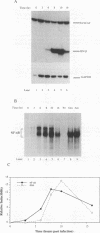
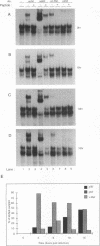
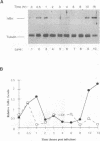
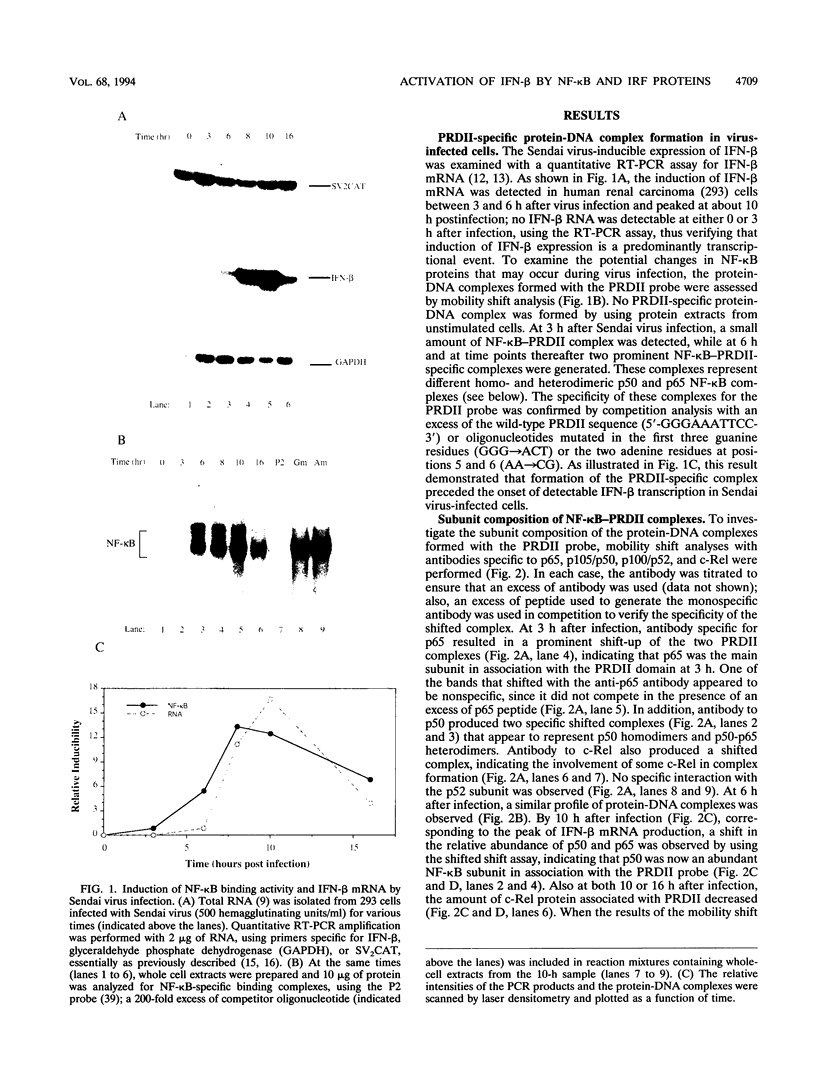
Multiple regulatory domains within the -100 region of the beta interferon (IFN-beta) promoter control the inducible response of the IFN gene to virus infection. In this study, we demonstrate that the formation of NF-kappa B-specific complexes on the positive regulatory domain II (PRDII) precedes the onset of detectable IFN-beta transcription in Sendai virus-infected cells. By using NF-kappa B subunit-specific antibodies, a temporal shift in the composition of NF-kappa B subunits in association with the PRDII domain is detected as a function of time after virus infection. Furthermore, a virus-induced degradation of I kappa B alpha (MAD3) protein is observed between 2 and 8 h after infection; at later times, de novo synthesis of I kappa B alpha restores I kappa B alpha to levels found in uninduced cells and correlates with the down regulation of IFN-beta transcription. In cotransfection experiments using various NF-kappa B subunit expression plasmids and two copies of PRDII/NF-kappa B linked to a chloramphenicol acetyltransferase reporter gene, we demonstrate that expression of p65, c-Rel, or p50 or combinations of p50-p65 and p65-c-Rel differentially stimulated PRDII-dependent transcription. Coexpression of I kappa B alpha completely abrogated p65-, c-Rel-, or p65-p50-induced gene activity. When the entire IFN-beta promoter (-281 to +19) was used in coexpression studies, synergistic stimulation of IFN-beta promoter activity was obtained when NF-kappa B subunits were coexpressed together with the IFN regulatory factor 1 (IRF-1) transcription factor. Overexpression of either I kappa B or the IRF-2 repressor was able to abrogate inducibility of the IFN-beta promoter. Thus, multiple regulatory events--including differential activation of DNA-binding NF-kappa B heterodimers, degradation of I kappa B alpha, synergistic interaction between IRF-1 and NF-kappa B, and decreased repression by I kappa B and IRF-2--are all required for the transcriptional activation of the IFN-beta promoter.
Full text
PDF

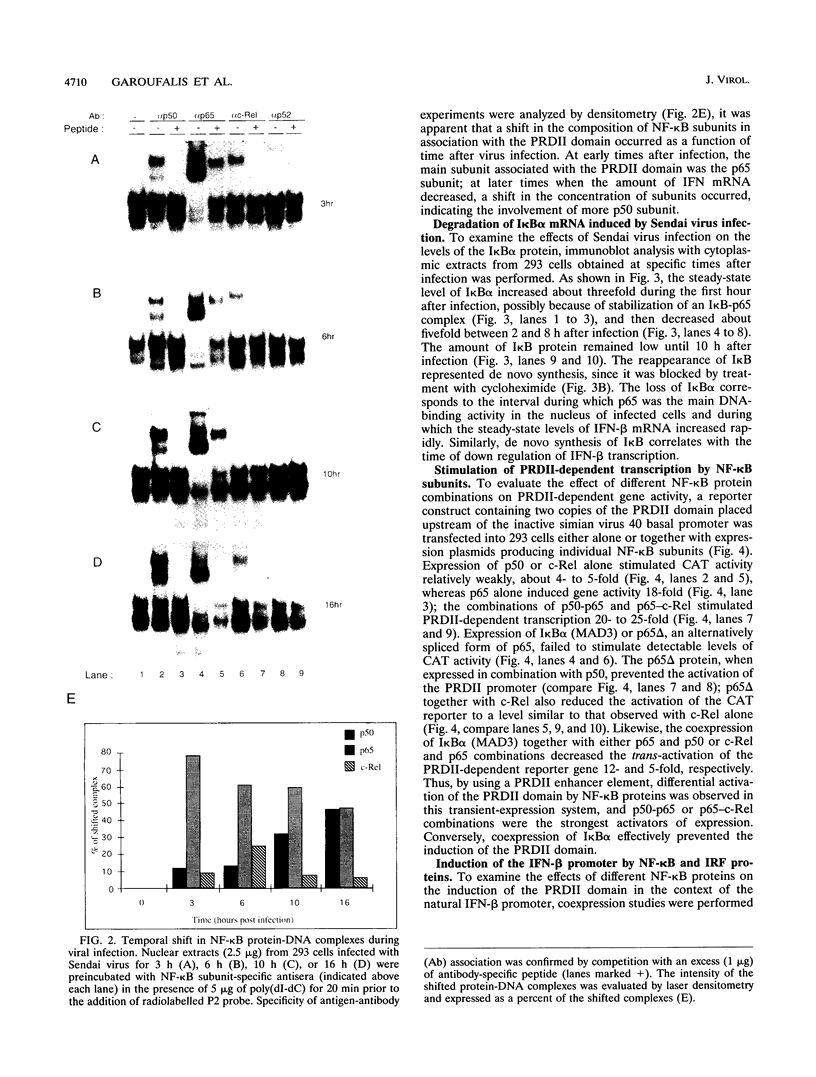
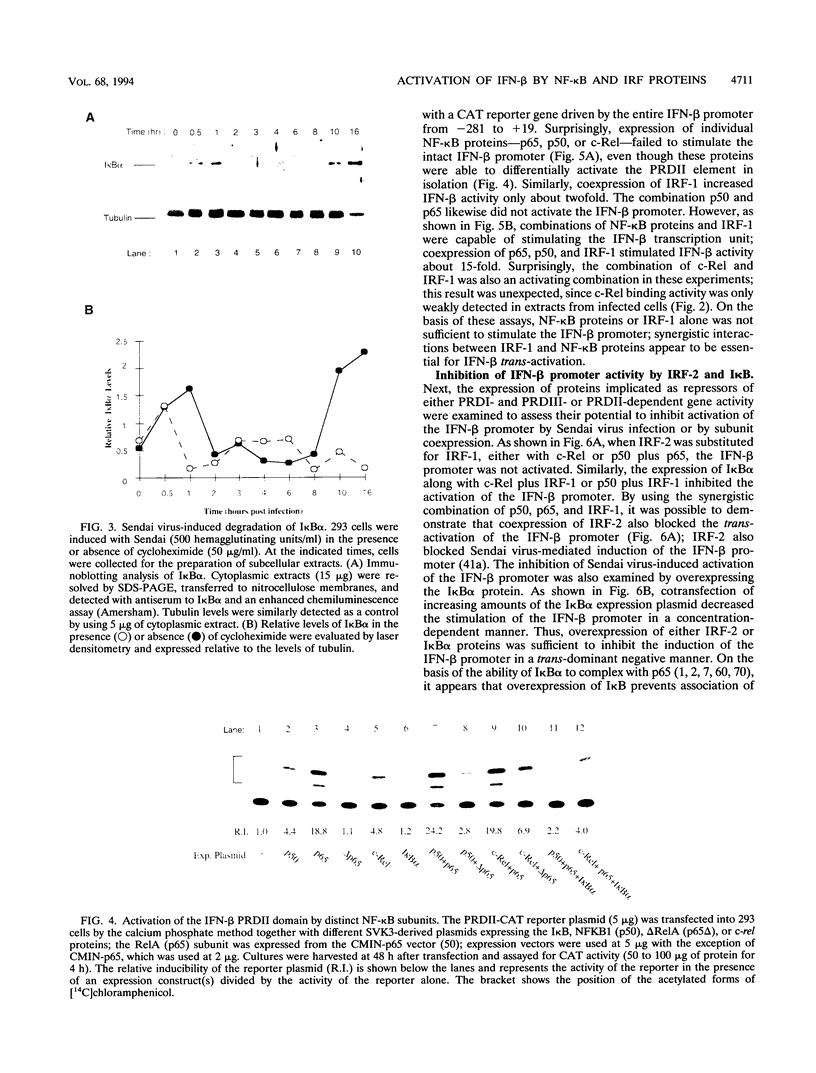
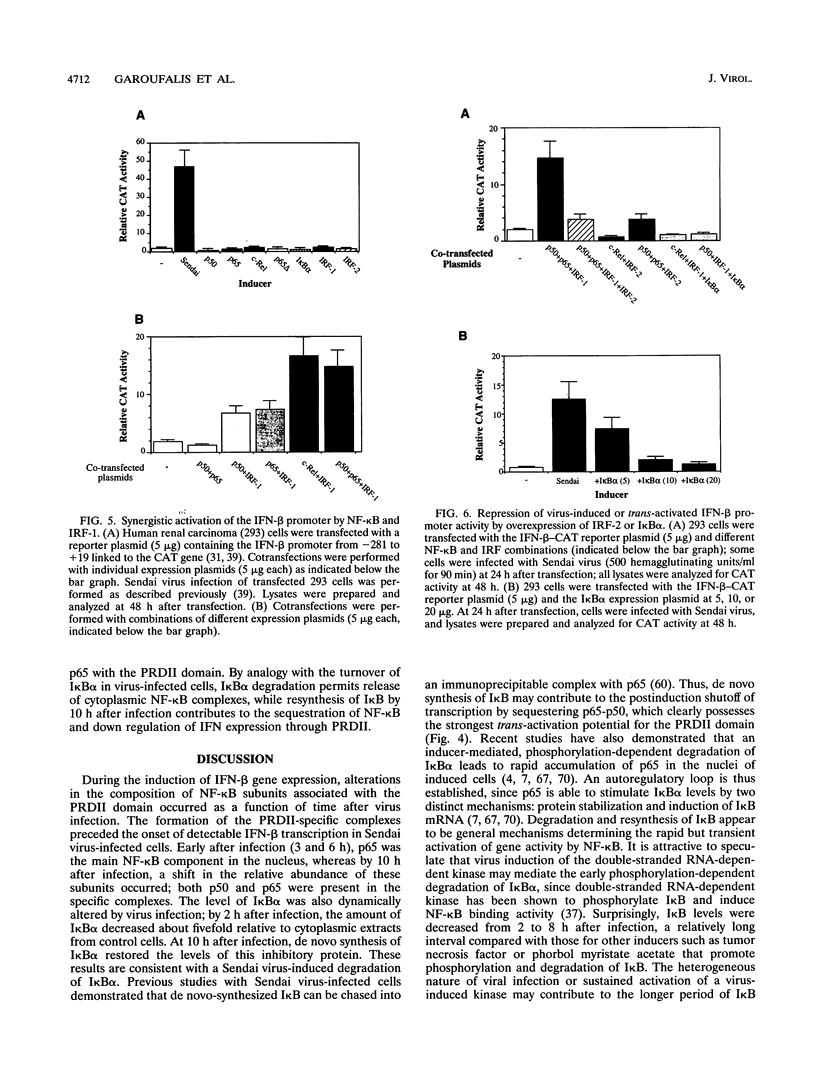



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev. 1989 Nov;3(11):1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Baldwin A. S., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993 Nov;7(11):2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993 Jun;13(6):3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A. A., Ruben S. M., Scheinman R. I., Haskill S., Rosen C. A., Baldwin A. S., Jr I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: a mechanism for cytoplasmic retention. Genes Dev. 1992 Oct;6(10):1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- Bours V., Villalobos J., Burd P. R., Kelly K., Siebenlist U. Cloning of a mitogen-inducible gene encoding a kappa B DNA-binding protein with homology to the rel oncogene and to cell-cycle motifs. Nature. 1990 Nov 1;348(6296):76–80. doi: 10.1038/348076a0. [DOI] [PubMed] [Google Scholar]
- Brown K., Park S., Kanno T., Franzoso G., Siebenlist U. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell E., Mittereder N., Rice N. R. A human rel proto-oncogene cDNA containing an Alu fragment as a potential coding exon. Oncogene. 1989 Jul;4(7):935–942. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cohen L., Hiscott J. Characterization of TH3, an induction-specific protein interacting with the interferon beta promoter. Virology. 1992 Dec;191(2):589–599. doi: 10.1016/0042-6822(92)90234-g. [DOI] [PubMed] [Google Scholar]
- Cohen L., Hiscott J. Heterodimerization and transcriptional activation in vitro by NF-kappa B proteins. J Cell Physiol. 1992 Jul;152(1):10–18. doi: 10.1002/jcp.1041520103. [DOI] [PubMed] [Google Scholar]
- D'Addario M., Roulston A., Wainberg M. A., Hiscott J. Coordinate enhancement of cytokine gene expression in human immunodeficiency virus type 1-infected promonocytic cells. J Virol. 1990 Dec;64(12):6080–6089. doi: 10.1128/jvi.64.12.6080-6089.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario M., Wainberg M. A., Hiscott J. Activation of cytokine genes in HIV-1 infected myelomonoblastic cells by phorbol ester and tumor necrosis factor. J Immunol. 1992 Feb 15;148(4):1222–1229. [PubMed] [Google Scholar]
- Du W., Maniatis T. An ATF/CREB binding site is required for virus induction of the human interferon beta gene [corrected]. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2150–2154. doi: 10.1073/pnas.89.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Thanos D., Maniatis T. Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell. 1993 Sep 10;74(5):887–898. doi: 10.1016/0092-8674(93)90468-6. [DOI] [PubMed] [Google Scholar]
- Fan C. M., Maniatis T. Generation of p50 subunit of NF-kappa B by processing of p105 through an ATP-dependent pathway. Nature. 1991 Dec 5;354(6352):395–398. doi: 10.1038/354395a0. [DOI] [PubMed] [Google Scholar]
- Fan C. M., Maniatis T. Two different virus-inducible elements are required for human beta-interferon gene regulation. EMBO J. 1989 Jan;8(1):101–110. doi: 10.1002/j.1460-2075.1989.tb03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Kimura Y., Miyamoto M., Barsoumian E. L., Taniguchi T. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature. 1989 Jan 19;337(6204):270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- Fujita T., Sakakibara J., Sudo Y., Miyamoto M., Kimura Y., Taniguchi T. Evidence for a nuclear factor(s), IRF-1, mediating induction and silencing properties to human IFN-beta gene regulatory elements. EMBO J. 1988 Nov;7(11):3397–3405. doi: 10.1002/j.1460-2075.1988.tb03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Shibuya H., Hotta H., Yamanishi K., Taniguchi T. Interferon-beta gene regulation: tandemly repeated sequences of a synthetic 6 bp oligomer function as a virus-inducible enhancer. Cell. 1987 May 8;49(3):357–367. doi: 10.1016/0092-8674(87)90288-1. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Maniatis T. Overlapping positive and negative regulatory domains of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1447–1451. doi: 10.1073/pnas.85.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty A., Camato R., Paterno G., Cohen L., Hiscott J., Skup D. A developmentally regulated octamer-binding activity in embryonal carcinoma cells which represses beta-interferon expression. Cell Growth Differ. 1991 Oct;2(10):503–510. [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989 Aug 25;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Harada H., Kitagawa M., Tanaka N., Yamamoto H., Harada K., Ishihara M., Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993 Feb 12;259(5097):971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- Harada H., Willison K., Sakakibara J., Miyamoto M., Fujita T., Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990 Oct 19;63(2):303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- Haskill S., Beg A. A., Tompkins S. M., Morris J. S., Yurochko A. D., Sampson-Johannes A., Mondal K., Ralph P., Baldwin A. S., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell. 1991 Jun 28;65(7):1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Henkel T., Zabel U., van Zee K., Müller J. M., Fanning E., Baeuerle P. A. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-kappa B subunit. Cell. 1992 Mar 20;68(6):1121–1133. doi: 10.1016/0092-8674(92)90083-o. [DOI] [PubMed] [Google Scholar]
- Hiscott J., Alper D., Cohen L., Leblanc J. F., Sportza L., Wong A., Xanthoudakis S. Induction of human interferon gene expression is associated with a nuclear factor that interacts with the NF-kappa B site of the human immunodeficiency virus enhancer. J Virol. 1989 Jun;63(6):2557–2566. doi: 10.1128/jvi.63.6.2557-2566.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., David M., Larner A. C., Finbloom D. S. In vitro activation of a transcription factor by gamma interferon requires a membrane-associated tyrosine kinase and is mimicked by vanadate. Mol Cell Biol. 1993 Jul;13(7):3984–3989. doi: 10.1128/mcb.13.7.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Kakizuka A., Verma I. M. I kappa B gamma, a 70 kd protein identical to the C-terminal half of p110 NF-kappa B: a new member of the I kappa B family. Cell. 1992 Mar 20;68(6):1109–1120. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- Keller A. D., Maniatis T. Identification and characterization of a novel repressor of beta-interferon gene expression. Genes Dev. 1991 May;5(5):868–879. doi: 10.1101/gad.5.5.868. [DOI] [PubMed] [Google Scholar]
- Keller A. D., Maniatis T. Identification of an inducible factor that binds to a positive regulatory element of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 May;85(10):3309–3313. doi: 10.1073/pnas.85.10.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Duckett C. S., Wamsley P., Zhang Q., Chiao P., Nabel G., McKeithan T. W., Baeuerle P. A., Verma I. M. The proto-oncogene bcl-3 encodes an I kappa B protein. Genes Dev. 1992 Dec;6(12A):2352–2363. doi: 10.1101/gad.6.12a.2352. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Lacoste J., D'Addario M., Roulston A., Wainberg M. A., Hiscott J. Cell-specific differences in activation of NF-kappa B regulatory elements of human immunodeficiency virus and beta interferon promoters by tumor necrosis factor. J Virol. 1990 Oct;64(10):4726–4734. doi: 10.1128/jvi.64.10.4726-4734.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClair K. P., Blanar M. A., Sharp P. A. The p50 subunit of NF-kappa B associates with the NF-IL6 transcription factor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8145–8149. doi: 10.1073/pnas.89.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc J. F., Cohen L., Rodrigues M., Hiscott J. Synergism between distinct enhanson domains in viral induction of the human beta interferon gene. Mol Cell Biol. 1990 Aug;10(8):3987–3993. doi: 10.1128/mcb.10.8.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Fan C. M., Maniatis T., Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989 Apr 21;57(2):287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- Liou H. C., Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993 Jun;5(3):477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- Liou H. C., Nolan G. P., Ghosh S., Fujita T., Baltimore D. The NF-kappa B p50 precursor, p105, contains an internal I kappa B-like inhibitor that preferentially inhibits p50. EMBO J. 1992 Aug;11(8):3003–3009. doi: 10.1002/j.1460-2075.1992.tb05370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald N. J., Kuhl D., Maguire D., Näf D., Gallant P., Goswamy A., Hug H., Büeler H., Chaturvedi M., de la Fuente J. Different pathways mediate virus inducibility of the human IFN-alpha 1 and IFN-beta genes. Cell. 1990 Mar 9;60(5):767–779. doi: 10.1016/0092-8674(90)90091-r. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Kimura T., Kitagawa M., Pfeffer K., Kawakami T., Watanabe N., Kündig T. M., Amakawa R., Kishihara K., Wakeham A. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 1993 Oct 8;75(1):83–97. [PubMed] [Google Scholar]
- Mercurio F., DiDonato J. A., Rosette C., Karin M. p105 and p98 precursor proteins play an active role in NF-kappa B-mediated signal transduction. Genes Dev. 1993 Apr;7(4):705–718. doi: 10.1101/gad.7.4.705. [DOI] [PubMed] [Google Scholar]
- Meyer R., Hatada E. N., Hohmann H. P., Haiker M., Bartsch C., Röthlisberger U., Lahm H. W., Schlaeger E. J., van Loon A. P., Scheidereit C. Cloning of the DNA-binding subunit of human nuclear factor kappa B: the level of its mRNA is strongly regulated by phorbol ester or tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):966–970. doi: 10.1073/pnas.88.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Klement J. F., Ruben S. M., Higgins K. A., Rosen C. A. Identification of a naturally occurring transforming variant of the p65 subunit of NF-kappa B. Science. 1992 Apr 17;256(5055):367–370. doi: 10.1126/science.256.5055.367. [DOI] [PubMed] [Google Scholar]
- Neri A., Chang C. C., Lombardi L., Salina M., Corradini P., Maiolo A. T., Chaganti R. S., Dalla-Favera R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell. 1991 Dec 20;67(6):1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Fujita T., Bhatia K., Huppi C., Liou H. C., Scott M. L., Baltimore D. The bcl-3 proto-oncogene encodes a nuclear I kappa B-like molecule that preferentially interacts with NF-kappa B p50 and p52 in a phosphorylation-dependent manner. Mol Cell Biol. 1993 Jun;13(6):3557–3566. doi: 10.1128/mcb.13.6.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan G. P., Ghosh S., Liou H. C., Tempst P., Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991 Mar 8;64(5):961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- Nourbakhsh M., Hoffmann K., Hauser H. Interferon-beta promoters contain a DNA element that acts as a position-independent silencer on the NF-kappa B site. EMBO J. 1993 Feb;12(2):451–459. doi: 10.1002/j.1460-2075.1993.tb05677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Takimoto G., McKeithan T. W. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990 Mar 23;60(6):991–997. doi: 10.1016/0092-8674(90)90347-h. [DOI] [PubMed] [Google Scholar]
- Palombella V. J., Maniatis T. Inducible processing of interferon regulatory factor-2. Mol Cell Biol. 1992 Aug;12(8):3325–3336. doi: 10.1128/mcb.12.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine R., Decker T., Kessler D. S., Levy D. E., Darnell J. E., Jr Purification and cloning of interferon-stimulated gene factor 2 (ISGF2): ISGF2 (IRF-1) can bind to the promoters of both beta interferon- and interferon-stimulated genes but is not a primary transcriptional activator of either. Mol Cell Biol. 1990 Jun;10(6):2448–2457. doi: 10.1128/mcb.10.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis L. F., Harada H., Wolchok J. D., Taniguchi T., Vilcek J. Critical role of a common transcription factor, IRF-1, in the regulation of IFN-beta and IFN-inducible genes. EMBO J. 1992 Jan;11(1):185–193. doi: 10.1002/j.1460-2075.1992.tb05041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., MacKichan M. L., Israël A. The precursor of NF-kappa B p50 has I kappa B-like functions. Cell. 1992 Oct 16;71(2):243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- Roulston A., Beauparlant P., Rice N., Hiscott J. Chronic human immunodeficiency virus type 1 infection stimulates distinct NF-kappa B/rel DNA binding activities in myelomonoblastic cells. J Virol. 1993 Sep;67(9):5235–5246. doi: 10.1128/jvi.67.9.5235-5246.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben S. M., Dillon P. J., Schreck R., Henkel T., Chen C. H., Maher M., Baeuerle P. A., Rosen C. A. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-kappa B. Science. 1991 Mar 22;251(5000):1490–1493. doi: 10.1126/science.2006423. [DOI] [PubMed] [Google Scholar]
- Ruben S. M., Klement J. F., Coleman T. A., Maher M., Chen C. H., Rosen C. A. I-Rel: a novel rel-related protein that inhibits NF-kappa B transcriptional activity. Genes Dev. 1992 May;6(5):745–760. doi: 10.1101/gad.6.5.745. [DOI] [PubMed] [Google Scholar]
- Ruben S. M., Narayanan R., Klement J. F., Chen C. H., Rosen C. A. Functional characterization of the NF-kappa B p65 transcriptional activator and an alternatively spliced derivative. Mol Cell Biol. 1992 Feb;12(2):444–454. doi: 10.1128/mcb.12.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Bull P., Takamiya M., Bours V., Siebenlist U., Dobrzanski P., Bravo R. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol Cell Biol. 1992 Feb;12(2):674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R. M., Perkins N. D., Duckett C. S., Andrews P. C., Nabel G. J. Cloning of an NF-kappa B subunit which stimulates HIV transcription in synergy with p65. Nature. 1991 Aug 22;352(6337):733–736. doi: 10.1038/352733a0. [DOI] [PubMed] [Google Scholar]
- Schmitz M. L., Baeuerle P. A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991 Dec;10(12):3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. L., Fujita T., Liou H. C., Nolan G. P., Baltimore D. The p65 subunit of NF-kappa B regulates I kappa B by two distinct mechanisms. Genes Dev. 1993 Jul;7(7A):1266–1276. doi: 10.1101/gad.7.7a.1266. [DOI] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987 Oct 30;238(4827):692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Sun S. C., Ganchi P. A., Ballard D. W., Greene W. C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993 Mar 26;259(5103):1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992 Nov 27;71(5):777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- Visvanathan K. V., Goodbourn S. Double-stranded RNA activates binding of NF-kappa B to an inducible element in the human beta-interferon promoter. EMBO J. 1989 Apr;8(4):1129–1138. doi: 10.1002/j.1460-2075.1989.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Sakakibara J., Hovanessian A. G., Taniguchi T., Fujita T. Activation of IFN-beta element by IRF-1 requires a posttranslational event in addition to IRF-1 synthesis. Nucleic Acids Res. 1991 Aug 25;19(16):4421–4428. doi: 10.1093/nar/19.16.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulczyn F. G., Naumann M., Scheidereit C. Candidate proto-oncogene bcl-3 encodes a subunit-specific inhibitor of transcription factor NF-kappa B. Nature. 1992 Aug 13;358(6387):597–599. doi: 10.1038/358597a0. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S., Cohen L., Hiscott J. Multiple protein-DNA interactions within the human interferon-beta regulatory element. J Biol Chem. 1989 Jan 15;264(2):1139–1145. [PubMed] [Google Scholar]
- Zabel U., Baeuerle P. A. Purified human I kappa B can rapidly dissociate the complex of the NF-kappa B transcription factor with its cognate DNA. Cell. 1990 Apr 20;61(2):255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]