Abstract
Epstein-Barr virus (EBV), a herpesvirus with oncogenic potential, is camouflaged with glycoprotein 350/220, which mimics the human ligand C3dg and thereby binds to and exploits complement receptor type 2 (CR2; CD21), the EBV receptor. It has not been possible to determine the role of CR2 during postbinding events of viral infection because all B lymphocytes express endogenous CR2, precluding an informative study of receptor mutants. We have overcome this obstacle through creation of a novel experimental system based on molecular dissection of the ligand-binding domains of human CR2 and murine CR2. Our results demonstrate first, that two discontinuous amino acid substitutions within the ligand-binding domain of murine CR2 render it capable of mediating EBV infection of human B-lymphoblastoid cells, and second, that the specific role of CR2 during EBV infection is to capture virions at the cell surface, after which cofactors not associated with CR2 mediate postbinding events. These are the first studies to be described in which a cell that is normally susceptible to viral infection can be manipulated so as to direct entry of virions via recombinant or endogenous receptors.
Full text
PDF


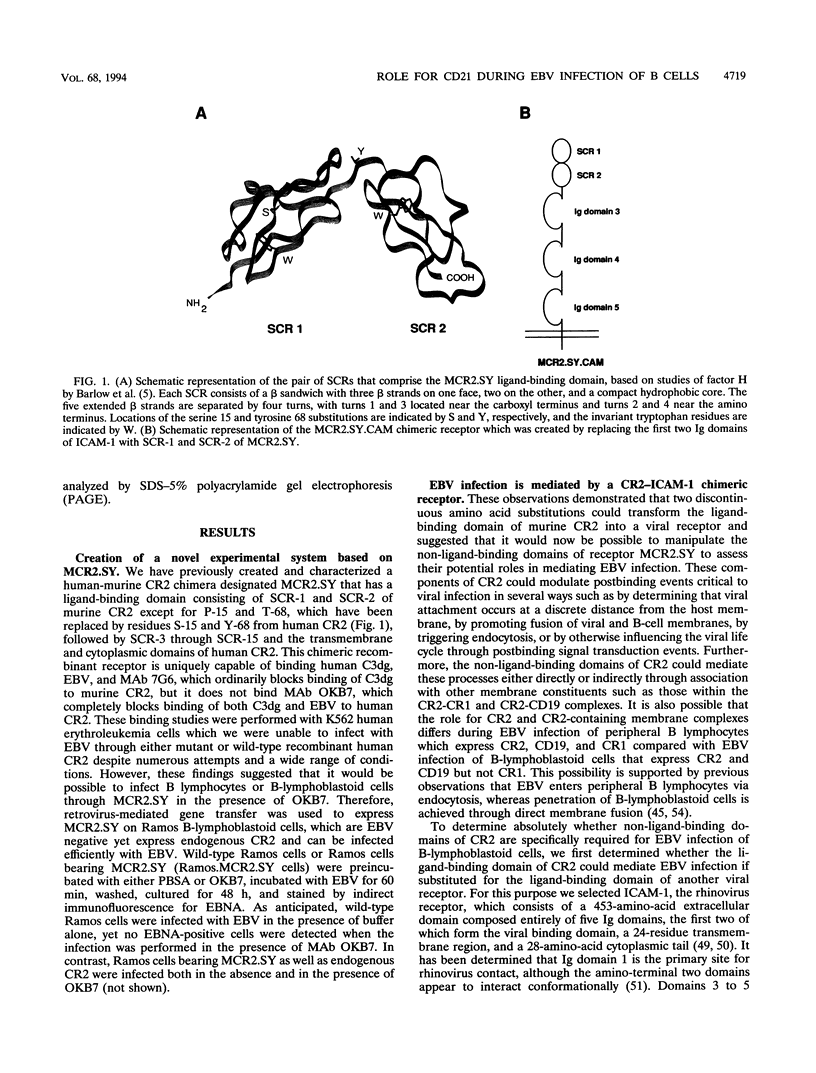
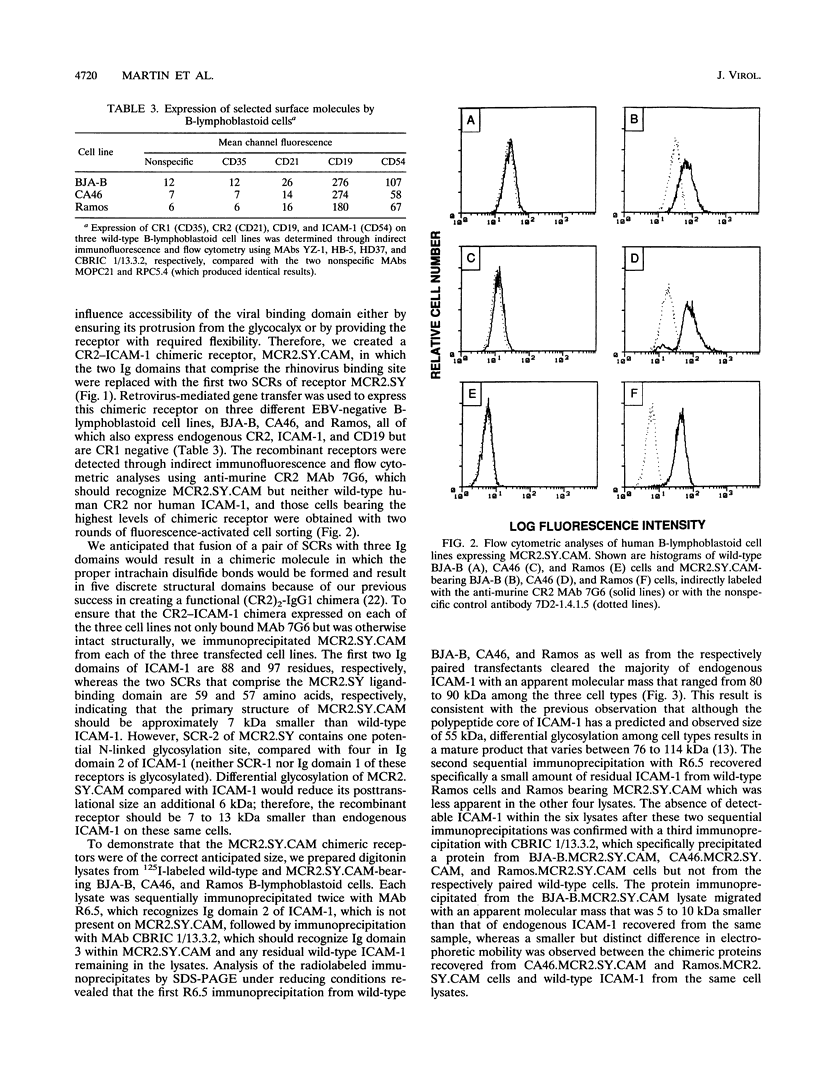

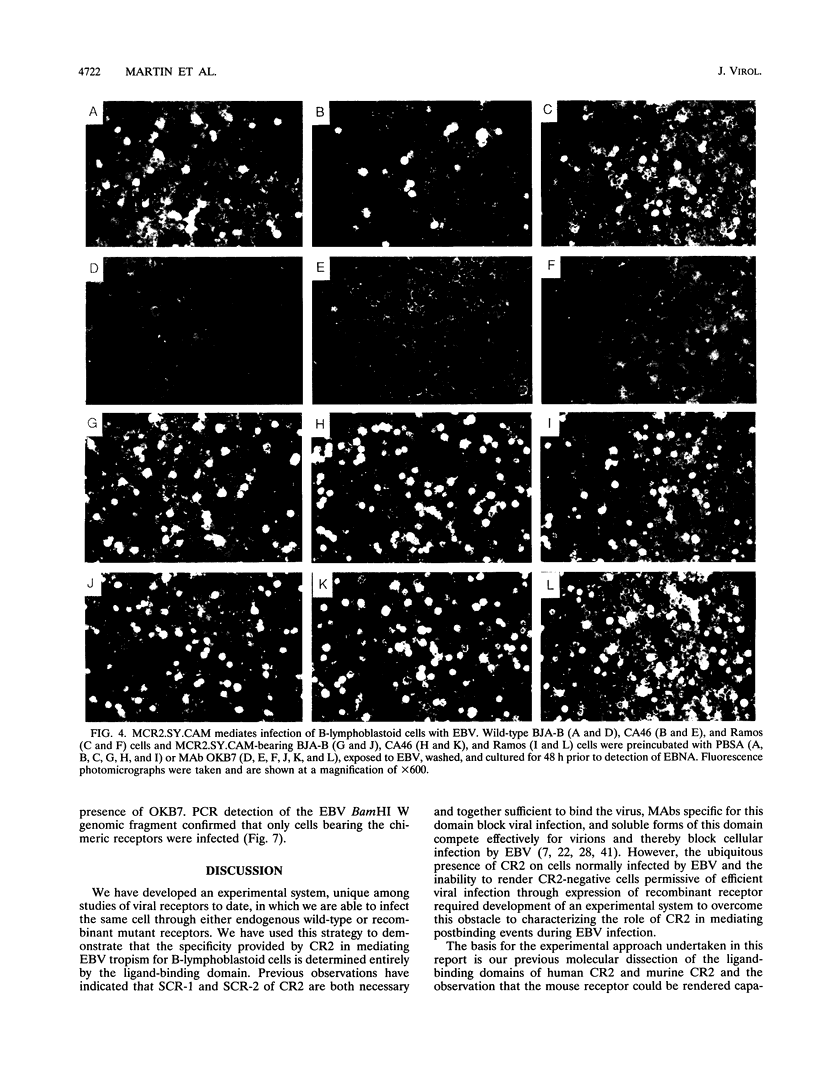
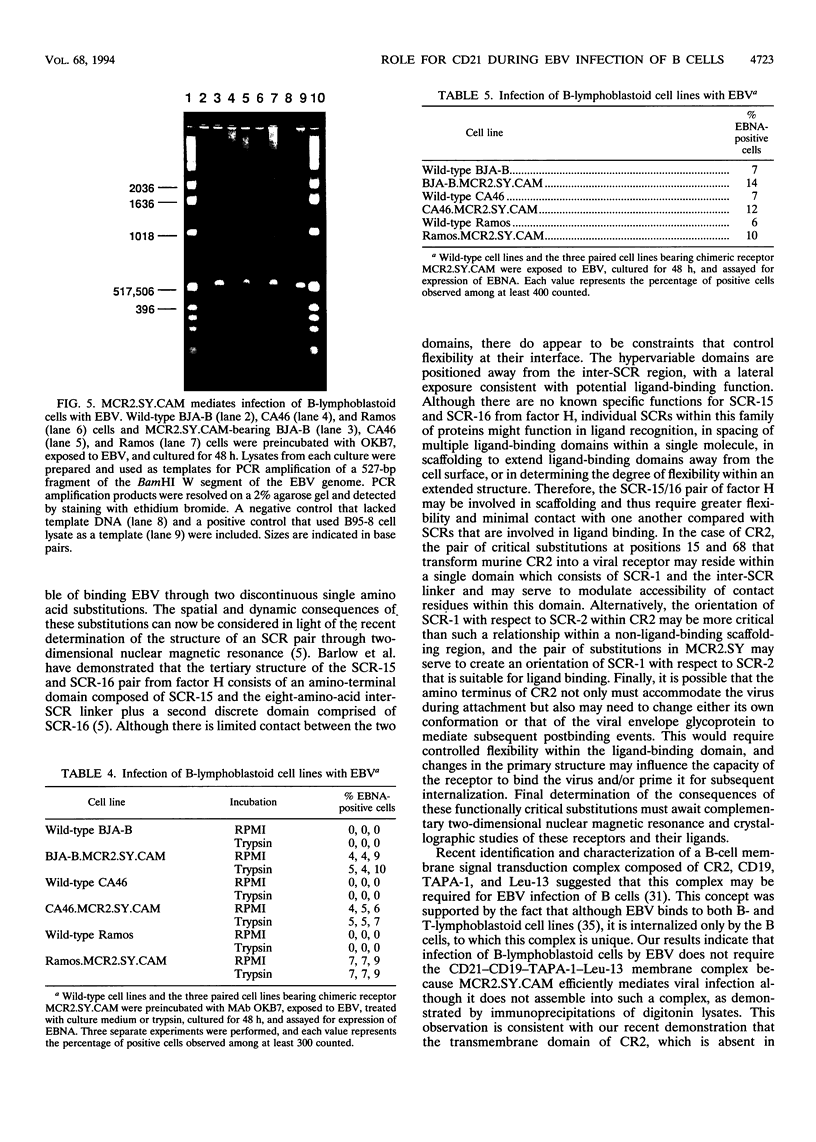


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn J. M., Fearon D. T. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21). Adv Immunol. 1989;46:183–219. doi: 10.1016/s0065-2776(08)60654-9. [DOI] [PubMed] [Google Scholar]
- Ahearn J. M., Hayward S. D., Hickey J. C., Fearon D. T. Epstein-Barr virus (EBV) infection of murine L cells expressing recombinant human EBV/C3d receptor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9307–9311. doi: 10.1073/pnas.85.23.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Barlow P. N., Norman D. G., Steinkasserer A., Horne T. J., Pearce J., Driscoll P. C., Sim R. B., Campbell I. D. Solution structure of the fifth repeat of factor H: a second example of the complement control protein module. Biochemistry. 1992 Apr 14;31(14):3626–3634. doi: 10.1021/bi00129a011. [DOI] [PubMed] [Google Scholar]
- Barlow P. N., Steinkasserer A., Norman D. G., Kieffer B., Wiles A. P., Sim R. B., Campbell I. D. Solution structure of a pair of complement modules by nuclear magnetic resonance. J Mol Biol. 1993 Jul 5;232(1):268–284. doi: 10.1006/jmbi.1993.1381. [DOI] [PubMed] [Google Scholar]
- Borisch B., Hennig I., Laeng R. H., Waelti E. R., Kraft R., Laissue J. Association of the subtype 2 of the Epstein-Barr virus with T-cell non-Hodgkin's lymphoma of the midline granuloma type. Blood. 1993 Aug 1;82(3):858–864. [PubMed] [Google Scholar]
- Carel J. C., Myones B. L., Frazier B., Holers V. M. Structural requirements for C3d,g/Epstein-Barr virus receptor (CR2/CD21) ligand binding, internalization, and viral infection. J Biol Chem. 1990 Jul 25;265(21):12293–12299. [PubMed] [Google Scholar]
- Changelian P. S., Jack R. M., Collins L. A., Fearon D. T. PMA induces the ligand-independent internalization of CR1 on human neutrophils. J Immunol. 1985 Mar;134(3):1851–1858. [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Cooper N. R., Moore M. D., Nemerow G. R. Immunobiology of CR2, the B lymphocyte receptor for Epstein-Barr virus and the C3d complement fragment. Annu Rev Immunol. 1988;6:85–113. doi: 10.1146/annurev.iy.06.040188.000505. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dörig R. E., Marcil A., Chopra A., Richardson C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993 Oct 22;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Lifson J. D. HIV interactions with CD4: a continuum of conformations and consequences. Immunol Today. 1992 Jun;13(6):201–206. doi: 10.1016/0167-5699(92)90154-Y. [DOI] [PubMed] [Google Scholar]
- Fingeroth J. D., Benedict M. A., Levy D. N., Strominger J. L. Identification of murine complement receptor type 2. Proc Natl Acad Sci U S A. 1989 Jan;86(1):242–246. doi: 10.1073/pnas.86.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingeroth J. D. Comparative structure and evolution of murine CR2. The homolog of the human C3d/EBV receptor (CD21). J Immunol. 1990 May 1;144(9):3458–3467. [PubMed] [Google Scholar]
- Fischer E., Delibrias C., Kazatchkine M. D. Expression of CR2 (the C3dg/EBV receptor, CD21) on normal human peripheral blood T lymphocytes. J Immunol. 1991 Feb 1;146(3):865–869. [PubMed] [Google Scholar]
- Fricks C. E., Hogle J. M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990 May;64(5):1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Lee W. C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992 Aug;66(8):5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Hebell T., Ahearn J. M., Fearon D. T. Suppression of the immune response by a soluble complement receptor of B lymphocytes. Science. 1991 Oct 4;254(5028):102–105. doi: 10.1126/science.1718035. [DOI] [PubMed] [Google Scholar]
- Janatova J., Reid K. B., Willis A. C. Disulfide bonds are localized within the short consensus repeat units of complement regulatory proteins: C4b-binding protein. Biochemistry. 1989 May 30;28(11):4754–4761. doi: 10.1021/bi00437a036. [DOI] [PubMed] [Google Scholar]
- Kanavaros P., Lescs M. C., Brière J., Divine M., Galateau F., Joab I., Bosq J., Farcet J. P., Reyes F., Gaulard P. Nasal T-cell lymphoma: a clinicopathologic entity associated with peculiar phenotype and with Epstein-Barr virus. Blood. 1993 May 15;81(10):2688–2695. [PubMed] [Google Scholar]
- Kinoshita T., Takeda J., Hong K., Kozono H., Sakai H., Inoue K. Monoclonal antibodies to mouse complement receptor type 1 (CR1). Their use in a distribution study showing that mouse erythrocytes and platelets are CR1-negative. J Immunol. 1988 May 1;140(9):3066–3072. [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Korbjuhn P., Anagnostopoulos I., Hummel M., Tiemann M., Dallenbach F., Parwaresch M. R., Stein H. Frequent latent Epstein-Barr virus infection of neoplastic T cells and bystander B cells in human immunodeficiency virus-negative European peripheral pleomorphic T-cell lymphomas. Blood. 1993 Jul 1;82(1):217–223. [PubMed] [Google Scholar]
- Lowell C. A., Klickstein L. B., Carter R. H., Mitchell J. A., Fearon D. T., Ahearn J. M. Mapping of the Epstein-Barr virus and C3dg binding sites to a common domain on complement receptor type 2. J Exp Med. 1989 Dec 1;170(6):1931–1946. doi: 10.1084/jem.170.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Martin D. R., Yuryev A., Kalli K. R., Fearon D. T., Ahearn J. M. Determination of the structural basis for selective binding of Epstein-Barr virus to human complement receptor type 2. J Exp Med. 1991 Dec 1;174(6):1299–1311. doi: 10.1084/jem.174.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A. K., Kopicky-Burd J., Carter R. H., Tuveson D. A., Tedder T. F., Fearon D. T. Intersection of the complement and immune systems: a signal transduction complex of the B lymphocyte-containing complement receptor type 2 and CD19. J Exp Med. 1991 Jan 1;173(1):55–64. doi: 10.1084/jem.173.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A. K., Martin D. R., Carter R. H., Klickstein L. B., Ahearn J. M., Fearon D. T. Functional dissection of the CD21/CD19/TAPA-1/Leu-13 complex of B lymphocytes. J Exp Med. 1993 Oct 1;178(4):1407–1417. doi: 10.1084/jem.178.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Menezes J., Seigneurin J. M., Patel P., Bourkas A., Lenoir G. Presence of Epstein-Barr virus receptors, but absence of virus penetration, in cells of an Epstein-Barr virus genome-negative human lymphoblastoid T line (Molt 4). J Virol. 1977 Jun;22(3):816–821. doi: 10.1128/jvi.22.3.816-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer W. J., Johnston R. E. Structural rearrangement of infecting Sindbis virions at the cell surface: mapping of newly accessible epitopes. J Virol. 1993 Sep;67(9):5117–5125. doi: 10.1128/jvi.67.9.5117-5125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miller N., Hutt-Fletcher L. M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988 Jul;62(7):2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina H., Kinoshita T., Inoue K., Carel J. C., Holers V. M. A molecular and immunochemical characterization of mouse CR2. Evidence for a single gene model of mouse complement receptors 1 and 2. J Immunol. 1990 Nov 1;145(9):2974–2983. [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Moore M. D., Cannon M. J., Sewall A., Finlayson M., Okimoto M., Nemerow G. R. Inhibition of Epstein-Barr virus infection in vitro and in vivo by soluble CR2 (CD21) containing two short consensus repeats. J Virol. 1991 Jul;65(7):3559–3565. doi: 10.1128/jvi.65.7.3559-3565.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. D., Cooper N. R., Tack B. F., Nemerow G. R. Molecular cloning of the cDNA encoding the Epstein-Barr virus/C3d receptor (complement receptor type 2) of human B lymphocytes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9194–9198. doi: 10.1073/pnas.84.24.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. D., DiScipio R. G., Cooper N. R., Nemerow G. R. Hydrodynamic, electron microscopic, and ligand-binding analysis of the Epstein-Barr virus/C3dg receptor (CR2). J Biol Chem. 1989 Dec 5;264(34):20576–20582. [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., Cooper N. R. Early events in the infection of human B lymphocytes by Epstein-Barr virus: the internalization process. Virology. 1984 Jan 15;132(1):186–198. doi: 10.1016/0042-6822(84)90102-8. [DOI] [PubMed] [Google Scholar]
- Norman D. G., Barlow P. N., Baron M., Day A. J., Sim R. B., Campbell I. D. Three-dimensional structure of a complement control protein module in solution. J Mol Biol. 1991 Jun 20;219(4):717–725. doi: 10.1016/0022-2836(91)90666-t. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Silver L., Anderson C. W. Interaction of human adenovirus serotype 2 with human lymphoid cells. Virology. 1988 Aug;165(2):377–387. doi: 10.1016/0042-6822(88)90582-x. [DOI] [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Erickson H. P., Springer T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990 Apr 20;61(2):243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Takada K., Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989 Jan;63(1):445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Goldmacher V. S., Lambert J. M., Schlossman S. F. Epstein Barr virus binding induces internalization of the C3d receptor: a novel immunotoxin delivery system. J Immunol. 1986 Aug 15;137(4):1387–1391. [PubMed] [Google Scholar]
- Thomas J. A., Cotter F., Hanby A. M., Long L. Q., Morgan P. R., Bramble B., Bailey B. M. Epstein-Barr virus-related oral T-cell lymphoma associated with human immunodeficiency virus immunosuppression. Blood. 1993 Jun 15;81(12):3350–3356. [PubMed] [Google Scholar]
- Tuveson D. A., Ahearn J. M., Matsumoto A. K., Fearon D. T. Molecular interactions of complement receptors on B lymphocytes: a CR1/CR2 complex distinct from the CR2/CD19 complex. J Exp Med. 1991 May 1;173(5):1083–1089. doi: 10.1084/jem.173.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J. J., Toothaker L. E., Smith J. A., Weis J. H., Fearon D. T. Structure of the human B lymphocyte receptor for C3d and the Epstein-Barr virus and relatedness to other members of the family of C3/C4 binding proteins. J Exp Med. 1988 Mar 1;167(3):1047–1066. doi: 10.1084/jem.167.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman H. F., Bartow T., Leppo M. K., Marsh H. C., Jr, Carson G. R., Concino M. F., Boyle M. P., Roux K. H., Weisfeldt M. L., Fearon D. T. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990 Jul 13;249(4965):146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]