Abstract
To investigate the clinical significance of circulating angiogenic factors, especially in association with early relapse of osteosarcoma, we quantified pre-therapeutic levels of vascular endothelial growth factor, basic fibroblast growth factor and placenta growth factor in the sera of 16 patients with osteosarcoma using an enzyme-linked immunosorbent assay. After a 1-year follow-up, the serum level of angiogenic factors was analysed with respect to microvessel density of the biopsy specimen and clinical disease relapse. The serum vascular endothelial growth factor levels were positively correlated with the microvessel density with statistical significance (P=0.004; Spearman rank correlation) and also significantly higher in seven patients who developed pulmonary metastasis than the remaining nine patients without detectable disease relapse (P=0.0009; The Mann–Whitney U-test). In contrast, the serum levels of basic fibroblast growth factor or placenta growth factor failed to show significant correlation with the microvessel density or relapse of the disease. Although there was no significant correlation between serum vascular endothelial growth factor levels and the tumour volume, the serum vascular endothelial growth factor levels were significantly higher in patients with a vascular endothelial growth factor-positive tumour than those with a vascular endothelial growth factor-negative tumour. These findings suggest that the pre-therapeutic serum vascular endothelial growth factor level reflects the angiogenic property of primary tumour and may have a predictive value on early disease relapse of osteosarcoma.
British Journal of Cancer (2002) 86, 864–869. DOI: 10.1038/sj/bjc/6600201 www.bjcancer.com
© 2002 Cancer Research UK
Keywords: osteosarcoma, pulmonary metastasis, angiogenesis, VEGF
Angiogenesis is an absolute requirement for the neoplastic growth of solid tumours after they reach a critical size of 1–2 mm3 (Folkman, 1995). It is also essential for tumour metastasis, facilitating the shedding of tumour cells into surrounding blood vessels (Folkman, 1971, 1972, 1990). Tumour cells have been shown to secrete a variety of angiogenic factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and placenta growth factor (PlGF) and thereby induce the local formation of new blood capillaries (Kandel et al, 1991; Ferrara et al, 1992; Dvorak et al, 1995). An association between poor prognosis and increases in vascularity has been reported in a number of tumours, including breast carcinoma (Weidner et al, 1991), lung carcinoma (Weidner et al, 1993), prostate carcinoma (Macchiarini et al, 1992), cervical carcinoma (Smith-McCune and Weidner, 1994) and colon carcinoma (Takahashi et al, 1995).
Osteosarcoma is one of the most common malignant bone tumours. Despite recent advances in multimodality treatments consisting of chemotherapy and wide tumour resection, pulmonary metastasis occurs in approximately 50% of the patients with osteosarcoma and remains a major cause of fatal outcome (Rosen et al, 1974). Notably, such relapses with pulmonary metastasis or deaths most likely occur during the first year of treatment (Eilber et al, 1987; Pratt et al, 1990; Souhami et al, 1997). Therefore, it is particularly important for the treatment of osteosarcoma to predict the relapse of the tumour at the early phase and customise the protocols.
We previously demonstrated that VEGF expression in osteosarcoma tumour tissue is correlated with high microvessel density, metastatic spread and poor prognosis (Kaya et al, 2000). Recently, VEGF has been measured not only in tissues but also in sera by using an enzyme-linked immunosorbent assay (ELISA), and increased serum levels of VEFG have been reported to be correlated with a high incidence of remote metastasis and a poor prognosis in patients with various types of cancer (Salven et al, 1997, 1998; Hyodo et al, 1998; Jin-no et al, 1998; Kumar et al, 1998; Landriscina et al, 1998; Hara et al, 1999; Oehler and Caffier, 2000). Measurement of serum VEGF levels by ELISA appears to be a more promising procedure for quantification than immunohistochemical staining of tissue VEGF, although VEGF can be secreted by megakaryocytes and platelets other than tumour cells (Banks et al, 1998; Webb et al, 1998; Salven et al, 1999; George et al, 2000; Lee et al, 2000).
In the present study, we investigated the clinical significance of serum VEGF, bFGF, and PlGF in osteosarcoma in a prospective manner, focusing on the correlation with early disease relapse and local angiogenesis. Serum VEGF levels were corrected by platelet counts and the relationship of serum VEGF with tissue VEGF expression as well as the volume of the primary tumours was also analysed.
MATERIALS AND METHODS
Patients and sample processing
This study was approved under the institutional guidelines for the use of human subjects in research. Between April 1998 and March 2000 (patients having presented in March 2001 could not be followed up for 1 year, thus the study duration should end in 2000 at latest), all consecutive patients with putative diagnosis of osteosarcoma gave informed consent to provide blood samples. Peripheral venous blood samples were taken from these patients before biopsy and the serum was stored at –80°C until the assays were performed. Among them, patients whose biopsy specimens exhibited histological features compatible to osteosarcoma were entered into the study. There were 16 patients (four women and 12 men) with the age ranging from 20 to 69 years (average 31.2 years). Nine of the tumours were located in the femur, four in the tibia, two in the humerus and one in the pelvis. All tumours were histologically high grade. None of the patients were associated with Paget's disease. Pretreatment work-up studies including palpation of the regional lymph nodes, plain chest X-rays, computed tomography of the lung and abdomen, and bone scintigraphy revealed the development of pulmonary metastasis in two patients. These 16 patients were enrolled into the treatment protocol consisting of neoadjuvant chemotherapy, wide tumour excision, and adjuvant chemotherapy, which was basically a combination of high-dose methotrexate, doxorubicine, cisplatin and ifosmaide. Wide resection margin was achieved in all patients.
Early relapse was defined as the detectable tumour developing in remote sites within 1 year from the onset of the treatments. The cases in which metastatic disease was evident at the onset of the treatments were also defined as early relapse. During a 1-year period, patients were taken plain chest X-rays every month and computed tomography of the lung every 3 months. Bone scintigraphy was taken at 1 year. Consequently, seven out of 16 patients showed early relapse of the disease, all of them being pulmonary metastasis. These patients were divided into two groups, the relapse (seven patients) and no-relapse group (nine patients).
Immunohistochemical staining
The expression of VEGF and CD34 in the biopsy specimens was determined using the avidin-biotin complex method as described previously (Kaya et al, 2000). The primary antibody for VEGF was a rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1 : 200 dilution, and the antibody for CD34 was a mouse monoclonal antibody (Nichirei, Tokyo, Japan) at 1 : 100 dilution. The polyclonal antibody reactivity for VEGF with individual tissue sections was considered positive if equivalent staining was seen either in the membrane or the cytoplasm of more than 30% of the tumour cells. The number of CD34-positive vessels was counted in four randomly selected areas of a 1-mm2 field and the average number was referred as the microvessel density.
Evaluation and statistical analysis
Concentration of VEGF, bFGF and PlGF in the sera of patients taken before biopsy was assessed by a commercially available sandwich ELISA (VEGF, IBL, Fujioka, Japan; bFGF and PlGF, R&D Systems, Minneapolis, MN, USA). The serum levels of each angiogenic factor were analysed with respect to (i) the correlation with microvessel density in the biopsy specimen and (ii) the difference between the relapse group and the no-relapse group. The serum VEGF level corrected for the number of platelet before biopsy was also included in the analysis. In addition, the correlation of the serum level of VEGF with (i) volume of the primary tumour and (ii) expression of VEGF in the biopsy specimens were analysed. The volume of the primary tumour was assessed upon the longitudinal and transverse images of magnetic resonance imaging that had been taken before biopsy, and calculated with the following formula: π/6×height×width×depth.
Spearman rank correlation test was used for the analysis of the correlation between the serum levels of each angiogenic factor and the microvessel density, between the serum VEGF level and the tumour volume, and between the serum VEGF level and patient age. The Mann–Whitney U-test was used for the comparative analysis of each angiogenic factor levels between the relapse group and no-relapse group and between the VEGF-positive group and the VEGF-negative group in evaluation of the biopsy specimen. Statistical significance was defined as P<0.05.
RESULTS
Clinical parameters of 16 patients with osteosarcoma
Table 1 summarises the clinical parameters of individual patients. The average serum levels and the 95% confidence interval (CI) of each angiogenic factor were following, VEGF; 1069.4 pg ml−1 (95% CI=551.0–2075.5 pg ml−1), bFGF; 109.7 pg ml−1 (95% CI=61.1–196.8 pg ml−1), PlGF; 60.0 pg ml−1 (95% CI=32.2–111.9 pg ml−1). To determine the distribution of values, means and confidence intervals on the log-transformed data have been back-transformed to give estimates on the untransformed scale.
Table 1. Patients and clinical parameters.
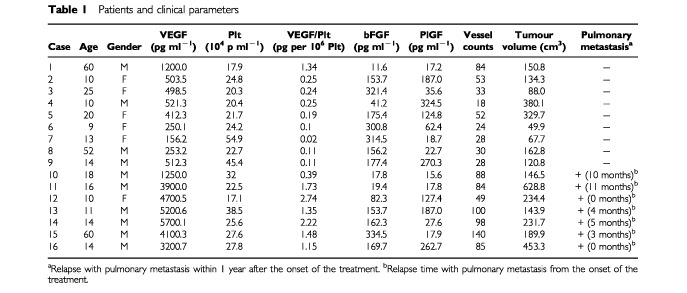
Two out of 16 patients had pulmonary metastases at the time of the diagnosis and five patients relapsed with pulmonary metastasis within 1 year after the onset of the treatments.
Serum angiogenic factors concentration and microvessel density
We first analysed the correlation between the serum levels of angiogenic factors and the microvessel density in the biopsy specimen of the primary tumour. As shown in Figure 1A, there was a significant correlation between serum VEGF level and vessel counts within the osteosarcoma tumour (Figure 1A, P=0.004; Spearman rank correlation). The correlation remained significant when the serum VEGF levels were corrected by platelet counts (Figure 1B, P=0.0057). In contrast, no statistically significant correlation was observed between the serum PlGF or serum bFGF level and microvessel density (Figure 1C, D, PlGF: P=0.169; bFGF: P=0.529). These results indicate that the serum VEGF level reflects the microvessel density of the primary osteosarcoma tumour. There was no correlation between the serum VEGF level and patient age (P=0.898).
Figure 1.
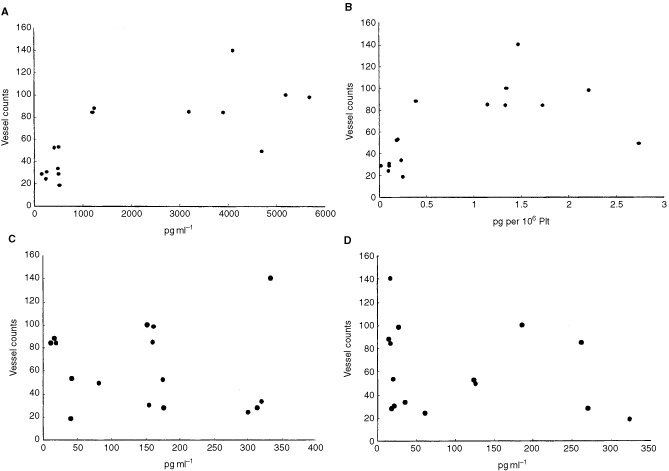
Relationship between microvessel count and serum angiogenic factors levels. Serum angiogenic factors levels against the vessel counts in 16 patients with osteosarcoma. (A) VEGF, (B) VEGF corrected for platelet counts (C) bFGF and (D) PlGF. The microvessel counts of the primary osteosarcoma tumours correlated with serum VEGF level (P=0.004). and serum VEGF/Plt level (P=0.0057). In contrast, no statistically significant correlation was observed between the serum PlGF or serum bFGF level and microvessel density.
Serum angiogenic factors concentration and early disease relapse
We next assessed the association between the serum angiogenic factor levels and early relapse of osteosarcoma. The serum VEGF level was significantly higher in the relapse group than that in the no-relapse group (Figure 2A, P=0.0009; Mann–Whitney U-test). The above-mentioned mean value of serum VEGF (1069.4 pg ml−1) was chosen as the cut-off category, yielding the sensitivity of 100% and the specificity of 88.9% for detecting early disease relapse. The significance was retained after the serum VEGF level was corrected for platelet counts (Figure 2B, P=0.0018). In contrast, there was no significant differences in the serum PlGF or serum bFGF level between the two groups (Figure 2C, D, PlGF: P=0.458; bFGF: P=0.397).
Figure 2.
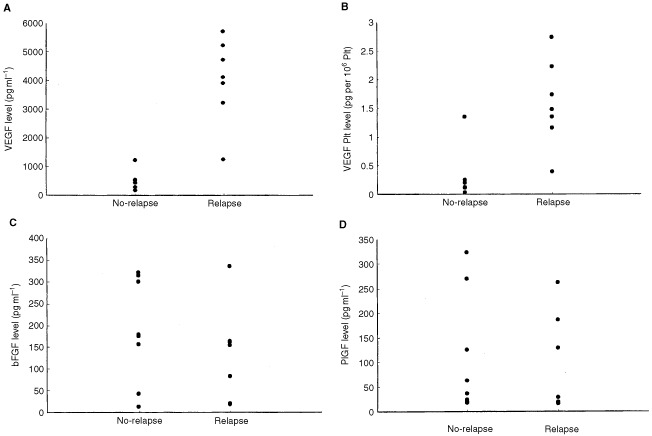
Relationship between the disease relapse and serum concentration of each angiogenic factor. The differences in each angiogenic factor level between the no-relapse and relapse group. The serum levels of VEGF and the serum levels of VEGF corrected for the count of platelet were significantly higher in the patients of the relapse group. In contrast, there was no significant differences in the serum PlGF or serum bFGF level between the two groups.
Serum VEGF levels, tumour volume andtissue VEGF expression
To clarify whether the serum level of VEGF is dependent on the quantitative or qualitative aspects of the primary tumour, we assessed the relationship between the serum VEGF level and the tumour volume as well as the tissue VEGF expression. As shown in Figure 3A, there was no significant correlation between the serum VEGF level and the tumour volume. In contrast, the serum VEGF levels were significantly higher in patients with a VEGF-positive tumour than those with a VEGF-negative tumour (Figure 3B, P=0.005). There was no significant differences in the tumour volume between the relapse and no-relapse groups (Figure 3C, P=0.064).
Figure 3.
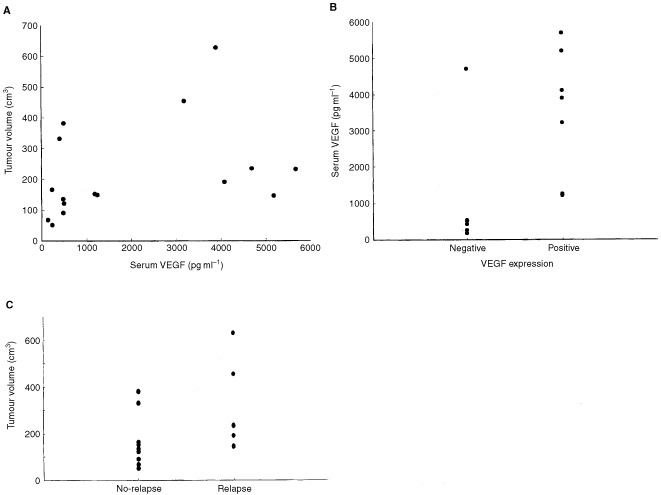
Relationship between the tumour volume or tumour VEGF expression serum VEGF level and disease relapse. (A) Relationship between the serum VEGF level and tumour volume. (B) Relationship between serum concentration of VEGF and in vivo expression of VEGF protein in the primary osteosarcoma tumour. (C) Relationship between the disease relapse and tumour volume.
DISCUSSION
In the current study, we have found that increased pre-therapeutic serum levels of VEGF in 16 patients with osteosarcoma correlate with (i) high microvessel density of the primary tumour, (ii) relapse with pulmonary metastasis during the first year of treatment, and (iii) positive expression of tissue VEGF. In contrast, serum bFGF or PlGF failed to show a positive correlation with the microvessel density or a significant association with relapse of the disease. These findings suggest that the pre-therapeutic serum VEGF levels reflect the angiogenic property of primary tumour and may have a predictive value on early disease relapse of osteosarcoma.
It is known that the majority of patients with osteosarcoma have micrometastatic diseases on initial presentation (Goorin et al, 1985; Eliber and Rosen, 1989), leading to early clinical relapse of the tumour in approximately 50% of the patients despite introduction of neoadjuvant chemotherapy (Rosen et al, 1974; Eilber et al, 1987; Pratt et al, 1990; Souhami et al, 1997). This fact implies the importance of identifying patients at high risk of relapse early in the course of disease, to whom a more intense chemotherapy or other therapeutic modalities are applied. Accordingly, an attempt has been made to switch the chemotherapy regimen into an alternative one with intensification of reagents before surgery in patients who exhibit progressive disease during neoadjuvant chemotherapy (Wada et al, 1996; Meyer et al, 2001). It appears to be more ideal to find a biologic profile of osteosarcoma, available at diagnosis, which is linked to relapse of the disease and poor prognosis. Measurement of pre-therapeutic serum VEGF is advantageous for its simple and rapid procedure and provides a prognostic value consistent with immunohistochemical (Kaya et al, 2000) or genetical detection (Lee et al, 1999) of tissue VEGF in biopsy specimens.
Recently, expression of VEGF has been examined in malignant bone and soft tissue tumours other than osteosarcoma and there have been contradictory results. Whereas Yudoh et al (2001) found a significant correlation between tissue VEGF expression and poor prognosis in soft tissue sarcomas, Chao et al (2001) and Kuhnen et al (2000) failed to find such significance. Kawauchi et al (1999) documented no prognostic significance of VEGF expression in synovial sarcoma. In chondrosarcomas, expression of VEGF was associated the histological grade (Ayala et al, 2000). Because histological grade is the most important, generally accepted, prognostic factor in soft tissue sarcomas as well as in chondrosarcoma, the predictive value of VEGF may be less important in those tumours than that in conventional osteosarcomas that are exclusively high grade in histology.
Contrary to VEGF, serum bFGF or PlGF measurements failed to show a significant correlation with the microvessel density or association with relapse of the disease, emphasising the critical role of VEGF in angiogenesis of osteosarcoma. Such a predominant role of VEGF than other angiogenic factors has been shown by comparative studies in gastric carcinoma (Yoshikawa et al, 2000) and renal cell carcinoma (Edgren et al, 1999). On the other hand, bFGF but not VEGF has shown prognostic relevance for head and neck cancer, suggesting that the dependency of tumoral neovascularisation on angiogenic factors may vary between tumour types (Dietz et al, 2000).
Since the values of serum VEGF in drawn blood samples can be increased during clot formation (Banks et al, 1998; Webb et al, 1998; Salven et al, 1999; George et al, 2000; Lee et al, 2000), it is possible that increased serum VEGF levels seen in the present study may not be a true reflection of tumour angiogenic activity. However, correction of the VEGF levels by platelet counts did not impair the significance in correlation with microvessel density or association with early disease relapse. In addition, the increased serum VEGF levels reflected well with tissue expression of VEGF protein within the primary tumour. It should be noted, in this regard, that one patient exhibited high serum VEGF despite negative tissue VEGF expression. In this patient, we have observed significant reduction of serum VEGF after removal of the primary tumour (Kaya M et al, unpublished observation), suggesting a problem in tissue staining procedure or the quality of the tissue sections examined.
Because of the rarity of osteosarcoma, there is a difficulty in designing a prospective study with a large number of participants. Although the median value of serum VEGF (1069.4 pg ml−1) chosen as the cut-off category yielded the sensitivity of 100% and the specificity of 88.9% for detecting early disease relapse, generalisation of this value requires additional prospective studies with large sample size as well as the sequential analysis of serum VEGF levels in individual patients at various points including the timing of disease relapse. In this sense, the present analysis serves as a pilot study with the strength in its prospective design and the uniformity of participants and treatment protocol.
In conclusion, the present study revealed the clinical significance of pre-therapeutic serum VEGF levels that were significantly higher in patients with osteosarcoma who relapsed during the first year of treatment, and also provided the basis to establish the anti-angiogenic principles for further therapy targeting patients at high risk of angiogenesis-dependent relapse of osteosarcoma.
Acknowledgments
We thank M Ono and M Naka for their excellent secretarial assistance, Dr T Akatsuka and Dr T Nosaka for technical assistance and MK Barrymore for comments on the manuscript. This work was supported in Grant-in-Aid 11307026 from the Ministry of Health and Welfare of Japan
References
- AyalaGLiuCNicosiaRHorowitzSLackmanR2000Microvasculature and VEGF expression in cartilaginous tumors Hum Pathol 31341346 [DOI] [PubMed] [Google Scholar]
- BanksREForbesMAKinseySEStanleyAInghamEWaltersCSelbyPJ1998Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology Br J Cancer 77956964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChaoCAl-SaleemTBrooksJJRogatkoAKraybillWGEisenbergB2001Vascular endothelial growth factor and soft tissue sarcomas: tumor expression correlates with grade Ann Surg Oncol 8260267 [DOI] [PubMed] [Google Scholar]
- DietzARudatVConradtCWeidauerHHoAMoehlerT2000Prognostic relevance of serum levels of the angiogenic peptide bFGF in advanced carcinoma of the head and neck treated by primary radiochemotherapy Head Neck 22666673 [DOI] [PubMed] [Google Scholar]
- DvorakHFBrownLFDetmarMDvorakAM1995Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis Am J Pathol 14610291039 [PMC free article] [PubMed] [Google Scholar]
- EdgrenMLennernasBLarssonANilssonS1999Serum concentrations of VEGF and b-FGF in renal cell, prostate and urinary bladder carcinomas Anticancer Res 19869873 [PubMed] [Google Scholar]
- EilberFGiulianoAEckardtJPattersonKMoseleySGoodnightJ1987Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial J Clin Oncol 52126 [DOI] [PubMed] [Google Scholar]
- EliberFRRosenG1989Adjuvant chemotherapy of osteosarcoma Semin Oncol 16312323 [PubMed] [Google Scholar]
- FolkmanJ1971Tumor angiogenesis: therapeutic implications N Engl J Med 28511821186 [DOI] [PubMed] [Google Scholar]
- FolkmanJ1972Anti-angiogenesis: new concept for therapy of solid tumors Ann Surg 17546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FolkmanJ1990What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 8246 [DOI] [PubMed] [Google Scholar]
- FolkmanJ1995Angiogenesis in cancer, vascular, rheumatoid and other disease Nat Med 12731 [DOI] [PubMed] [Google Scholar]
- FerraraNHouckLJakemanLLeungDW1992Molecular and biological properties of the vascular endothelial cell growth factor family of proteins Endoc Rev 131832 [DOI] [PubMed] [Google Scholar]
- GeorgeMLEcclesSATuttonMGAbulafiAMSwiftRI2000Correlation of plasma and serum vascular endothelial growth factor levels with platelet count in colorectal cancer: clinical evidence of platelet scavenging? Clin Cancer Res 631473152 [PubMed] [Google Scholar]
- GoorinAMAbelsonHTFreiIIIE1985Osteosarcoma: fifteen years later New Engl J Med 31316371642 [DOI] [PubMed] [Google Scholar]
- HaraIMiyakeHYamanakaKHaraSArakawaSKamidonoS1999Expression of CD44 adhesion molecules in nonpapillary renal cell carcinoma and normal kidneys Urology 54562566 [DOI] [PubMed] [Google Scholar]
- Jin-noKTanimizuMHyodoINishikawaYHosokawaYDoiTEndoHYamashitaTOkadaY1998Circulating vascular endothelial growth factor (VEGF) is a possible tumor marker for metastasis in human hepatocellular carcinoma J Gastroenterol 33376382 [DOI] [PubMed] [Google Scholar]
- HyodoIDoiTEndoHHosokawaYNishikawaYTanimizuMJinnoKKotaniY1998Clinical significance of plasma vascular endothelial growth factor in gastrointestinal cancer Eur J Cancer 3420412045 [DOI] [PubMed] [Google Scholar]
- KandelJBossy-WetzeERadvanyiFKlagsbrunMFolkmanFHanahanD1991Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma Cell 6610951104 [DOI] [PubMed] [Google Scholar]
- KawauchiSFukudaTTsuneyoshiM1999Angiogenesis does not correlate with prognosis or expression of vascular endothelial growth factor in synovial sarcomas Oncol Report 6959964 [DOI] [PubMed] [Google Scholar]
- KayaMWadaTAkatsukaTKawaguchiSNagoyaSShindohMHigashinoFMezawaFOkadaFIshiiS2000Vascular endothelial growth factor (VEGF) expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis Clin Cancer Res 6572577 [PubMed] [Google Scholar]
- KuhnenCLehnhardtMTolnayEMuehlbergerTVogtPMMullerKM2000Patterns of expression and secretion of vascular endothelial growth factor in malignant soft-tissue tumours J Cancer Res Clin Oncol 126219225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KumarHHeerKLeePWDuthieGSMacDonaldAWGreenmanJKerinMJMonsonJR1998Preoperative serum vascular endothelial growth factor can predict stage in colorectal cancer Clin Cancer Res 412791285 [PubMed] [Google Scholar]
- LandriscinaMCassanoARattoCLongoRIppolitiMPalazzottiBCrucittiFBaroneC1998Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer Br J Cancer 78765770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeeJKHongYJHanCJHwangDYHongSI2000Clinical usefulness of serum and plasma vascular endothelial growth factor in cancer patients: which is the optimal specimen? Int J Oncol 17149152 [PubMed] [Google Scholar]
- LeeYHTokunagaTOshikaYSutoRYanagisawaKTomisawaMFukudaHNakanoHAbeSTateishiAKijimaHYamazakiHTamaokiNUeyamaYNakamuraM1999Cell-retained isoforms of vascular endothelial growth factor (VEGF) are correlated with poor prognosis in osteosarcoma Eur J Cancer 3510891093 [DOI] [PubMed] [Google Scholar]
- MacchiariniPFontaniniGHardinMJSquartiniFAngelettiCA1992Relation of neovascularisation to metastasis of non-small-cell lung cancer Lancet 340145146 [DOI] [PubMed] [Google Scholar]
- MeyerWHPrattCBPoquetteCARaoBNParhamDMMarinaNMRappoASMahmoudHHJenkinsJJHarperJMeelMFletcherBD2001Carboplatin/Isofamide window therapy for osteosarcoma: results of the St Jude Children's Research Hospital OS-91 trial J Clin Oncol 19171182 [DOI] [PubMed] [Google Scholar]
- OehlerMKCaffierH2000Prognostic relevance of serum vascular endothelial growth factor in ovarian cancer Anticancer Res 2051095112 [PubMed] [Google Scholar]
- PrattCBChampionJEFlemingIDRaoBKumarAPEvansWEGreenAAGeorgeS1990Adjuvant chemotherapy for osteosarcoma of the extrimity: long-term results of two consecutive prospective protocol studies Cancer 65439445 [DOI] [PubMed] [Google Scholar]
- RosenGSuwansirikulSKwonCTanCWuSJBeattieJrEJMurphyML1974High-dose methotrexate with citrovorum factor rescue and Adriamycin in childhood osteogenic sarcoma Cancer 3311511163 [DOI] [PubMed] [Google Scholar]
- SalvenPTeerenhoviLJoensuuH1997A high pretreatment serum vascular endothelial growth factor concentration is associated with poor outcome in non-Hodgkin's lymphoma Blood 9031673172 [PubMed] [Google Scholar]
- SalvenPRuotsalainenTMattsonKJoensuuH1998High pre-treatment serum level of vascular endothelial growth factor (VEGF) is associated with poor outcome in small-cell lung cancer Int J Cancer 79144146 [DOI] [PubMed] [Google Scholar]
- SalvenPOrpanaAJoensuuH1999Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor Clin Cancer Res 5487491 [PubMed] [Google Scholar]
- Smith-McCuneKKWeidnerN1994Demonstration and characterization of the angiogenic properties of cervical dysplasia Cancer Res 54800804 [PubMed] [Google Scholar]
- SouhamiRLCraftAWVan der EijkenJWNooijMSpoonerDBramwellVHCWierzbickiRMalcolmAJKirkpatrickAUscinskaBMVan GlabbekeMMachinD1997Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European osteosarcoma intergroup Lancet 350911917 [DOI] [PubMed] [Google Scholar]
- TakahashiYKitadaiYBucanaCDClearyKREllisLM1995Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer Cancer Res 5539643968 [PubMed] [Google Scholar]
- WadaTIsuKTakedaNUsuiMIshiiSYamawakiS1996A preliminary report of neoadjuvant chemotherapy NSH-7 study in osteosarcoma: preoperative salvage chemotherapy based on clinical tumor response and the use of granulocyte colony-stimulating factor Oncology 53221227 [DOI] [PubMed] [Google Scholar]
- WebbNJBottomleyMJWatsonCJBrenchleyPE1998Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease Clin Sci (Lond) 94395404 [DOI] [PubMed] [Google Scholar]
- WeidnerNSempleJPWelchWRFolkmanJ1991Tumor angiogenesis and metastasis correlation in invasivebreast carcinoma N Engl J Med 32418 [DOI] [PubMed] [Google Scholar]
- WeidnerNCarrollPRFlaxJBlumenfeldWFolkmanJ1993Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma Am J Pathol 143401409 [PMC free article] [PubMed] [Google Scholar]
- YoshikawaTTsuburayaAKobayashiOSairenjiMMotohashiHYanomaSNoguchiY2000Plasma concentrations of VEGF and bFGF in patients with gastric carcinoma Cancer Lett 29712 [DOI] [PubMed] [Google Scholar]
- YudohKKanamoriMOhmoriKYasudaTAokiMKimuraT2001Concentration of vascular endothelial growth factor in the tumour tissue as a prognostic factor of soft tissue sarcomas Br J Cancer 8416101615 [DOI] [PMC free article] [PubMed] [Google Scholar]


