Abstract
The major vault protein is the main component on multimeric vault particles, that are likely to play an essential role in normal cell physiology and to be associated with multidrug resistance of tumour cells. In order to unravel the function of vaults and their putative contribution to multidrug resistance, specific antibodies are invaluable tools. Until now, only conventional major vault protein-reactive murine monoclonal antibodies have been generated, that are most suitable for immunohistochemical analyses. The phage display method allows for selection of human antibody fragments with potential use in clinical applications. Furthermore, cDNA sequences encoding selected antibody fragments are readily identified, facilitating various molecular targeting approaches. In order to obtain such human Fab fragments recognising major vault protein we used a large non-immunized human Fab fragment phage library. Phages displaying major vault protein-reactive Fabs were obtained through several rounds of selection on major vault protein-coated immunotubes and subsequent amplification in TG1 E coli bacteria. Eventually, one major vault protein-reactive clone was selected and further examined. The anti-major vault protein Fab was found suitable for immunohistochemical and Western blot analysis of tumour cell lines and human tissues. BIAcore analysis showed that the binding affinity of the major vault protein-reactive clone almost equalled that of the murine anti-major vault protein Mabs. The cDNA sequence of this human Fab may be exploited to generate an intrabody for major vault protein-knock out studies. Thus, this human Fab fragment should provide a valuable tool in elucidating the contribution(s) of major vault protein/vaults to normal physiology and cellular drug resistance mechanisms.
British Journal of Cancer (2002) 86, 954–962. DOI: 10.1038/sj/bjc/6600159 www.bjcancer.com
© 2002 Cancer Research UK
Keywords: LRP, MVP, multidrug resistance, Fab, phage display.
Multidrug resistance (MDR; reviewed in Schneider et al, 1999) is a phenomenon frequently observed in tumour cells that are unresponsive to anti-cancer drugs. Proteins which can be involved in this process are the ATP-binding Cassette (ABC) transporter proteins (Higgins, 1992) P-glycoprotein (P-gp, ABCB1; reviewed in Ambudkar et al, 1999), multidrug resistance protein 1 (MRP1, ABCC1), MRP2 (ABCC2), MRP3 (ABCC3; reviewed in Borst et al, 2000) and breast cancer resistance protein (BCRP, ABCG2; Doyle et al, 1998), as well as the lung resistance-related protein (LRP; Scheffer et al, 2000). LRP was identified as the major vault protein (MVP), the main component of vaults (Scheffer et al, 1995).
Vaults are large multimeric barrel-shaped particles that are highly conserved through evolution, and likely to play an essential role in normal cell physiology. They are composed of multiple copies of three proteins (240, 193 and 104 kDa in size) and unique untranslated RNA species. The 104 kDa subunit is termed the MVP as it constitutes >70% of the particle mass. The p240 minor vault protein is identical to the telomerase-associated protein (TEP1) (Kickhoefer et al, 1999a), a structural protein in the telomerase complex. The other high molecular weight minor vault protein, p193, is a poly (ADP-ribose) polymerase (PARP) and is therefore named VPARP, for vault-PARP (Kickhoefer et al, 1999b).
The hollow structure of vaults is consistent with a role in either the compartmentalisation of macromolecular assemblies or in subcellular transport. In multidrug resistant tumour cells up-regulation of human LRP/MVP mRNA and protein as well as vault particle copy number was observed (Scheper et al, 1993; Laurencot et al, 1997; Kickhoefer et al, 1998). Primary evidence for a direct causal relationship between LRP/MVP expression and drug resistance was provided by Kitazono et al (1999). They showed that reduction of LRP/MVP expression by use of LRP/MVP-specific ribozymes in a cell line induced to overexpress LRP/MVP by exposing cells to sodium butyrate was enough to prevent drug resistance. Still, these studies have to be confirmed by independent investigations.
In order to further study the function of vaults and their contribution to MDR, specific antibodies are essential. Several MVP-reactive murine monoclonal antibodies (Mabs) are now available. These Mabs were all produced by classical hybridoma technology. In the past years the technology of display on filamentous phage in combination with selection was developed into a powerful tool for the identification of antibodies of human origin (Marks et al, 1991; Winter et al, 1994; Hoogenboom et al, 1998). Partially or completely human antibodies are of particular interest as they are expected to elicit no or minimal immune responses when administered to patients. For the construction of antibody libraries, V-genes are amplified from B-cell cDNA, and heavy and light chain genes are randomly combined and cloned to encode a combinatorial library of single-chain variable fragments (scFv) or Fab antibody fragments (Clackson et al, 1991; Hoogenboom et al, 1991). Large non-immune human antibody libraries, made for example from the variable region genes of the B-cells of healthy individuals, have been used to select antibodies to a large panel of antigens, including self-, non-immunogenic- and relatively toxic antigens (Marks et al, 1991; de Haard et al, 1999). When using the phage display system, the cDNA of antibodies of interest becomes available without further cloning steps. In this study we used a large non-immunised human Fab fragment phage library described by de Haard et al (1999) to isolate a human Fab reactive to the MVP.
MATERIALS AND METHODS
Escherichia coli strain
Escherichia coli TG1: K12, D(lac-pro), supE, thi, hsdD5/F' traD36, proA+B+, laclq, lacZDM15.
Selection of phages displaying anti-MVP human Fab
For the selection of phages expressing anti-MVP Fab fragments a large non-immunised human Fab fragment phage library was used which was described by de Haard et al (1999). The library contains cDNA sequences, encoding highly diverse Fab fragments (3.7×1010) with additional c-myc and His tags, cloned in the phagemid vector pCES1.
Selection of phages was essentially according to published methods (Marks et al, 1991) on immunotubes (Nunc maxisorp; Life Technologies, Merelbeke, Belgium) coated overnight at 37°C with 20 μg recombinant full-length MVP (Schroeijers et al, 2000) in 2 ml coating buffer (0.05 M sodium carbonate, pH 9.6). Unbound antigen was removed and the tubes were blocked for 1 h with PBS/2% skimmed milk powder/0.05% Tween 20 (MT–PBS). One ml of the page stock solution containing approximately 1×1013 phages was diluted 1 : 1 in MT–PBS and allowed to bind to the antigen-coated immunotube for 2 h at room temperature. Unbound phages were washed off by 20 washes with PBS/0.05% Tween 20 followed by 20 washes with PBS. Antigen-bound phages were eluted off, using 1 ml freshly prepared 100 mM triethylamine (TEA) for 10 min. The solution was neutralised by adding 0.5 ml of Tris buffer (1 M, pH 7.4). The solution was incubated for another hour at room temperature on immunotubes coated only with block buffer, to remove aspecifically bound phages. Unbound, antigen-specific phages were used to infect exponentially growing (OD600 of 0.5) TG1 bacteria, for 30 min at 37°C. After infection, the bacteria were allowed to grow overnight at 30°C and glycerol stocks were made. To have an indication of the phage input/output ratio, titrations of the bacteria, immediately after infection, were plated on selective 2×TY (16 g l−1 trypton, 10 g l−1 yeast extract and 5 g l−1 NaCl) plates supplemented with 2% glucose and 100 μg ml−1 ampicillin. The plates were incubated overnight at 30°C and colonies were counted the following day. For the next selection round, a sufficient amount of bacteria to ensure the presence of at least 10 bacteria of each clone, was inoculated in 50 ml 2×TY medium containing 100 μg ml−1 ampicillin and 2% glucose, giving a start OD600 of approximately 0.05. Five ml of bacteria grown at 37°C to an OD600 of 0.5 were infected with 2 μl M13-K07 helper phage (approximately 1×1013 phages per ml) for 30 min at 37°C. The bacteria were spun down, resuspended in 50 ml of 2×TY medium containing 100 μg ml−1 ampicillin and 25 μg ml−1 kanamycin to select for super-infected bacteria, and grown overnight at 30°C. The bacteria were spun down, the selected phages secreted into the supernatant were precipitated twice using 1/5th volume 20% PEG 6000; 2.5 M NaCl, resuspended in 1 ml of PBS and used in the next selection round on antigen coated immunotubes. A total of four, essentially identical selection rounds was carried out on MVP-coated immunotubes.
Screening of individual phage clones
To select individual phage clones displaying MVP-reactive Fabs, single bacterial colonies were grown at small-scale overnight at 30°C in 2×TY/2% glucose/100 μg ml−1 ampicillin in U-shaped 96-well plates (Costar, Corning, NY, USA). These plates were kept as ‘master plates’ at −80°C after adding glycerol to a final concentration of 15%. Then, 1 : 100 dilutions of the bacterial colonies were grown for 2 h at 37°C in V-shaped 96-well plates (Costar). Phages displaying recombinant Fab were produced by adding 25 μl of 1 : 250 diluted helper phage stock solution. Supernatants containing phages were tested for reactivity in recombinant MVP coated ELISA plates. ELISA plates were coated overnight at 37°C with 100 μl, 5 μg MVP per ml coating buffer (0.05 M sodium carbonate, pH 9.6). Plates were rinsed, blocked with 200 μl PBS/1% BSA/0.05% Tween 20 and incubated with phage-containing supernatant for 1 h at room temperature. Bound phages were detected using an in-house produced guinea pig-anti-phage polyclonal antiserum (1 : 1000) and HRP-labelled swine–anti-guinea pig antiserum (1 : 500, Dako, Copenhagen, Denmark). Colour development was with 5-amino-2-hydroxybenzoic acid (5-AS; Merck, Darmstadt, Germany) and 0.02% H2O2 as a chromogen.
Large-scale production of Fab fragments
Large-scale induction of soluble Fabs from individual clones was performed on a 50 ml scale in 2×TY medium, containing 100 μg ml−1 ampicillin and 2% glucose. The bacteria were grown at 37°C to an OD600 of 0.9. The cells were pelleted, resuspended in 50 ml 2×TY, containing 100 μg ml−1 ampicillin and 1 mM isopropyl-1-thio-β-D-galactopyranoside (IPTG) to activate the LacZ promoter, and grown for 4 h at 30°C. Soluble Fabs were purified making use of the His-tag on Ni-NTA-superflow agarose beads according to the manufacturer's protocol (Qiagen Inc., CA, USA).
BstNI fingerprinting
Individual bacterial colonies were used for standard 40 cycle hot-start PCR reactions, using fd-tet-seq24 (5′-TTT GTC GTC TTT CCA GAC GTT AGT-3′) as a forward and M13-reverse (5′-AGC GGA TAA CAA TTT CAC ACA GG-3′) as a reverse primer. The resulting amplimers were digested with BstNI and loaded onto a 2% agarose gel. The banding pattern of the clones was analysed on a UV transluminator.
Sequence analysis
Plasmids from individual bacterial clones were sequenced using the dideoxy sequencing method on a semi-automated sequencer (Alf Express, Pharmacia, Uppsala, Sweden). CH1 for (5′-GTC CTT GAC CAG GCA GCC CAG GGC-3′) and M13 reverse (5′-AGC GGA TAA CAA TTT CAC ACA GG-3′) were used as forward and reverse primers, respectively. V-gene sequences were aligned to V-BASE (http://www.mrc-cpe.cam.ac.uk/imt-doc/public/INTRO.html).
Immunoprecipitation
Cells were trypsinised and lysed in PBS containing 1% NP40, 1 mM EDTA, 1 mM phenylmethylsulphonyl fluoride (PMSF) for 2 min at room temperature, followed by a boost with an ultra-sound finger. Large fragments and nuclei were removed by centrifugation at 9000 g. Clear supernatant was pre-incubated for 30 min with protein A-sepharose beads to reduce non-specific binding of labelled protein. Cell lysate supernatants or solutions containing approximately 10 μg recombinant MVP were incubated with 10 μl soluble Fab or, as a control, 10 μg of LRP-56 MAb 2 h at room temperature. To the Fab precipitation 100 μl of anti-c-myc mouse monoclonal antibody 9E10 was added and incubation was continued for another hour. Then 50 μl of prot A-sepharose beads (∼25 μl ‘packed beads’) was added and precipitation was allowed for 1 h. Precipitates were washed three times in lysis buffer and three times in PBS. Antibody–antigen interaction was broken by dispensing the beads in Western blot loading buffer, containing 200 mM Tris-HCl (pH 6.8), 1% β-mercaptoethanol, 8% SDS, 10% glycerol and 0.05% bromophenol blue.
Western blot analysis
Total cell lysates were made as described (Zaman et al, 1994). Protein concentrations were determined with a BioRad protein assay (BioRad, Richmond, CA, USA). Cell lysates (10–40 μg) or 2 μg recombinant MVP or immunoprecipitates were fractionated on a 8% polyacrylamide slab gel and transferred onto a nitrocellulose membrane by electroblotting. After blocking, the membrane was incubated for 2 h with anti-MVP Fab in an appropriate dilution. HRP-labelled rabbit–anti-human lambda chain (1 : 200, Dako) was used as a secondary antibody. Colour development was with 0.5 g l−1 3,3′-diaminobenzidine tetrahydrochloride, 0.15 g l−1 chloronaphthol and 0.02% H2O2 in PBS.
BIAcore analysis
The affinity of the anti-MVP antibodies was further examined by surface plasmon resonance on a BIAcore 2000 instrument (Biacore AB, Uppsala, Sweden). Recombinant fusion protein of MVP and glutathione-S-transferase (GST–MVP; Schroeijers et al, 2000) was immobilised on the flow cell of an activated CM-5 sensorchip according to the manufacturer's protocol (Biacore AB), yielding a surface of 2000 resonance units. Soluble Fab fragments and control murine Mabs were analysed at room temperature, at a flow rate of 25 μl per minute, using HBS-EP (Biacore AB, 0.01 M HEPES, pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% polysorbate 20) as a buffer. Dissociation rates were calculated from the sensorgrams using the BIA-evaluation software (Biacore AB).
Immunohistochemistry
Cytospin preparations and cryosections (4 μm) were air dried overnight and fixed at room temperature for 7 min in 100% acetone or for 10 min in 3% paraformaldehyde/0.3% glucose. For detection of MVP with MVP-37 Mab and human Fab MVP-φ4, the slides were pretreated with 6 N guanidine hydrochloride Fab MVP-φ4, the slides were pretreated with 6 N guanidine hydrochloride (GdnHCl) in 50 mM Tris-HCl (pH 7.5) for 10 min at room temperature, as described (Schroeijers et al, 2000). The slides were incubated with primary antibody for 1 h at room temperature or at 37°C for the GdHCl treated slides. Biotinylated rabbit-anti-mouse (1 : 150, Zymed, San Francisco, CA, USA) and HRP-labelled streptavidin (1 : 500, Zymed) were used as secondary reagents for the mouse Mabs. For detection of the human Fab, 1 : 10 diluted anti-c-myc-tag 9E10 Mab, and HRP-labelled rabbit-anti-mouse serum (1 : 500, Dako, Copenhagen, Denmark) was used.
Colour development was with 0.4 mg ml−1 amino-ethyl-carbazole (AEC) and 0.02% H2O2 as a chromogen.
Cell lines
The lung cancer cell lines SW1573 (parent), SW1573/2R120 (MVP and MRP1 positive), SW1573/2R160 (MDR1 P-gp positive and a low percentage of MVP positive cells) were described in reference (Kuiper et al, 1990). The lung cancer cell lines GLC4 (parent) and GLC4/ADR (MVP and MRP1 positive) were described in reference (Zijlstra et al, 1987). All cell lines were grown in Dulbecco's modified essential medium (Gibco Europe, Paisley, Scotland), supplemented with 10% heat-inactivated foetal calf serum, 2 mM L-glutamine, penicillin and streptomycin. Resistant cell lines were cultured in the presence of drugs until 3–10 days before the experiments. All cells were negative for Mycoplasma as tested by the Gene-Probe rapid Mycoplasma detection system (Gene-Probe, San Diego, CA, USA).
Antibodies
Murine anti-MVP Mabs used were LRP-56 (Scheper et al, 1993), LMR-5 (Flens et al, 1997), MVP-9, MVP-16, MVP-37 (Schroeijers et al, 2001) and LRP (Transduction Laboratories, Lexington, KY, USA).
The anti-c-myc mouse Mab 9E10 was described in Chan et al (1987). The guinea pig-anti-phage polyclonal antiserum was produced by immunizing guinea pigs with wild type M13-K07 helper phages suspended in Freund's complete adjuvant (Difco, Detroit, MI, USA). Two booster injections with phages in PBS were given. Serum was collected and used without further purification.
RESULTS
Selection of anti-MVP human Fab
The successive rounds to select anti-MVP human Fabs on immunotubes coated with recombinant MVP, showed a gradual increase in the amount of antigen bound phages (output number). When the output number of round one was set to 1, output numbers of 50, 500 and 10 000 were noted for round 2, 3 and 4, respectively (Table 1). Apparently, the cycles of selection and re-amplication yielded increasing numbers of antigen-binding phages. ELISA screening of approximately 750 individual clones from round 2, 3 and 4, resulted in the identification of several MVP-reactive phages. The percentage of antigen binding phages per selection round is depicted in Table 1. Twenty-two individual MVP-reactive clones from round 3 and 4 were selected for BstNI fingerprinting. These clones all showed a very similar banding pattern, that was strikingly different from a randomly selected non-reactive clone (Figure 1). Sequence analysis of six of these clones confirmed that they were all identical. One clone, named MVP-φ4, was used for further characterization studies. The sequences of the heavy and light chain variable regions of the MVP-φ4 clone are available under accession numbers AJ306838 and AJ306837, respectively, at the EMBL database.
Table 1. Enrichment of MVP binding phages per selection round.
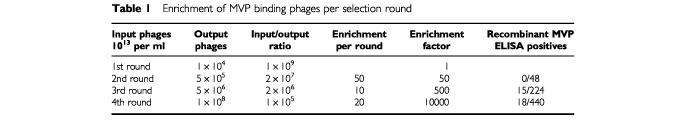
Figure 1.
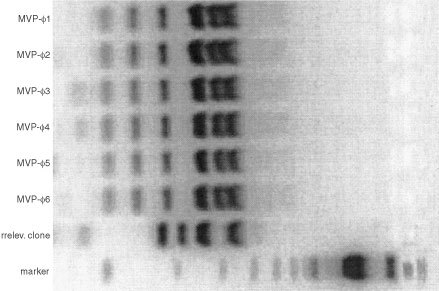
BstNI fingerprinting of six MVP-reactive clones from selection rounds 3 and 4 and a randomly selected non-reactive clone. cDNA of individual clones was PCR amplified, BstNI digested and loaded on an agarose gel. The MVP-reactive clones all show a very similar banding pattern, strikingly different from the irrelevant clone.
Heavy and light chain usage of the anti-MVP Fab
Comparison of the obtained cDNA sequence of the anti-MVP Fab with V-BASE showed that the Fab uses a heavy chain belonging to the VH4 family and a light chain belonging to the VL6 family (Figures 2 and 3). Highest homology was observed to germline clones DP-66/V71–2…+ (heavy chain) and 6a.366F5/V1–22…+ (light chain). The antigen specific CDR3 sequences for the heavy and light chain are: QGVYYYYGMDV and QSYSNNKWI, respectively. The number of amino acid changes from the germline, excluding the CDR3 region, is eight for the heavy chain and 10 for the light chain.
Figure 2.
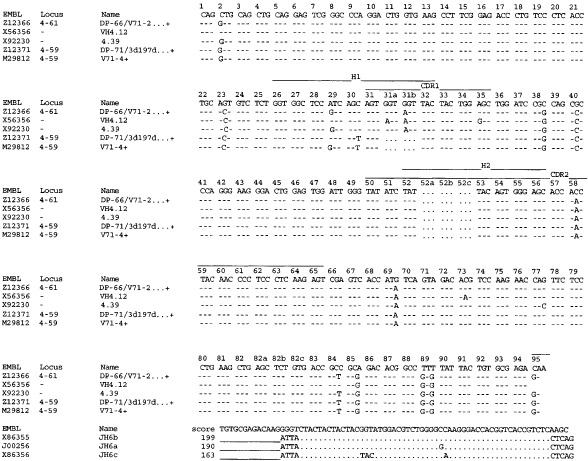
Comparison of the obtained heavy chain cDNA sequence of the anti-MVP Fab MVP-φ4 (accession number AJ306838) with V-BASE. The Fab uses a heavy chain belonging to the VH4 family. Highest homology was observed to germline clone DP-66/V71-2…+. The antigen specific CDR3 sequences for the heavy chain are: QGVYYYYGMDV. The number of amino acid changes from the germline, excluding the CDR3 region, is eight.
Figure 3.
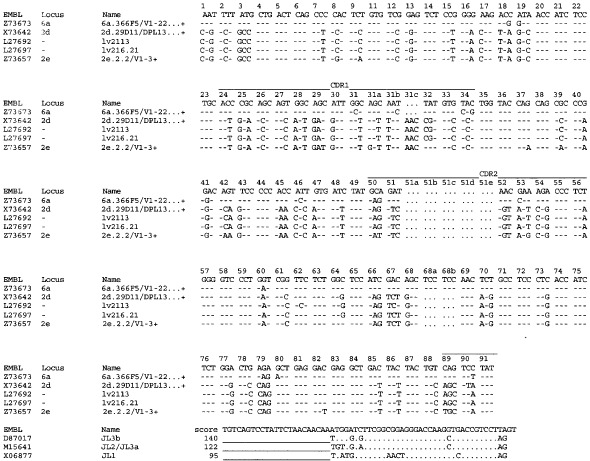
Comparison of the obtained light chain cDNA sequence of the anti-MVP Fab MVP-φ4 (accession number AJ306837) with V-BASE. The Fab uses a light chain belonging to the VL6 family. Highest homology was observed to germline clone 6a.366F5/V1-22…+. The antigen specific CDR3 sequences for the light chain are: QSYSNNKWI. The number of amino acid changes from the germline, excluding the CDR3 region, is 10.
Reactivity of anti-MVP Fab in Western blots and immunoprecipitation
In Western blots of cell lysates of MVP-overexpressing GLC4 ADR cells, MVP-φ4 was reactive with the MVP protein at the expected Mr of 110 000 (Figure 4A). Furthermore, MVP-φ4 was able to detect the Mr110 000 immunoprecipitation product of the anti-MVP mouse Mab LRP-56 and a cell lysate of the GLC4 ADR cells in the same test system (Figure 4B). Also, the recombinant MVP could be detected in similar Western blot experiments (not shown).
Figure 4.
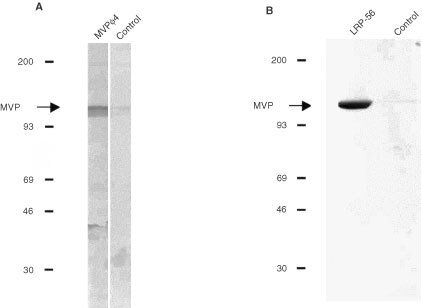
Western blots of cell lysates of MVP-overexpressing GLC4 ADR cells (A) and the immunoprecipitation products of the anti-MVP mouse Mab LRP-56 (B; left lane) or a control Mab (B; right lane) from a cell lysate of the GLC4 ADR cells. Proteins were loaded, separated and blotted. Blots were incubated with MVP-φ4 (A, left lane and B) or a control Fab (A, right lane) followed by HRP-labelled rabbit-anti-human lambda chain. Colour development was with DAB/chloronaphthol/H2O2. On both blots, MVP-φ4 was reactive with the MVP protein at the expected Mr of 110 000 (arrow).
In immunoprecipitation experiments, the anti-MVP Fab was unable to precipitate the MVP protein from either MVP-overexpressing GLC4 ADR cells or recombinant MVP in solution (not shown). These results indicate that MVP-φ4 is reactive to a linear or more denatured epitope of the MVP molecule. Of note, the LRP-56 mouse Mab is reactive to a more native epitope, judged from its reactivity in the immunoprecipitation experiments (see Figure 4B), and its inability to react with MVP in Western blots. Also, LRP-56 is unreactive with recombinant MVP coated in ELISA plates (not shown).
BIAcore analysis of anti-MVP Mabs and MVP-φ4
The off-rates of several murine anti-MVP Mabs and MVP-φ4 were determined by surface plasmon resonance on a BIAcore 2000 instrument, with recombinant GST–MVP immobilised on the flow cell of a CM-5 sensorchip, giving a surface of 2000 resonance units. As anticipated, the LRP-56 and LMR-5 Mabs were unreactive with MVP presented in this way, but the other available (bivalent) murine Mabs MVP-9, MVP-16, MVP-37, LRP were reactive with the MVP molecule immobilised on the chip (Figure 5). These, and the monovalent MVP-φ4 human Fab, gave off-rates of 1.3×10−3, 1.4×10−2, 1.3×10−4, 5.2×10−5, and 1.3×10−3, s−1, respectively.
Figure 5.
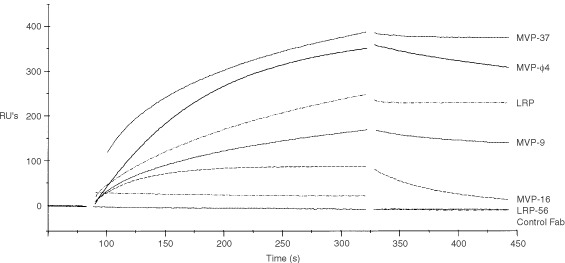
BIAcore analysis of the anti-MVP reagents. The off-rate of several murine anti-MVP Mabs and MVP-φ4 was determined by surface plasmon resonance on a BIAcore 2000 instrument, with recombinant GST-MVP immobilised on the flow cell of a CM-5 sensorchip. The control Fab and LRP-56 Mab (and LMR-5, not shown) were un-reactive in this system. The other murine Mabs MVP-9, MVP-16, MVP-37, LRP and the MVP-φ4 human Fab showed differential reactivity to the MVP molecule immobilised on the chip.
Reactivity of anti-MVP Fab on cytospins and tissue sections
A panel of cell lines with known presence or absence of MVP was stained with MVP-φ4. MVP-φ4 was only very moderately reactive on preparations of cell lines fixed with 100% acetone. However, using the guanidine fixation method (see Materials and methods), which facilitates detection of more denatured epitopes, the anti-MVP Fab showed the presence of MVP in GLC4 ADR cells, SW1573/2R120 cells and in an earlier identified small subpopulation of cells of the MDR1 P-gp positive SW1573/2R160 cell line (Figure 6A). Essentially similar results were obtained in control stainings with MVP-37, using the same treatment, or with LRP-56 Mab on acetone fixed cytospins. Further confirmation of the specificity of the anti-MVP Fab was obtained in studying human tissue staining. Using guanidine-treated, frozen sections of human lung tissue, MVP-φ4 was found to be reactive with MVP in the epithelial cells of the bronchi (Figure 6B). Again, comparable staining results were seen in the control stainings with MVP-37 and LRP-56.
Figure 6.
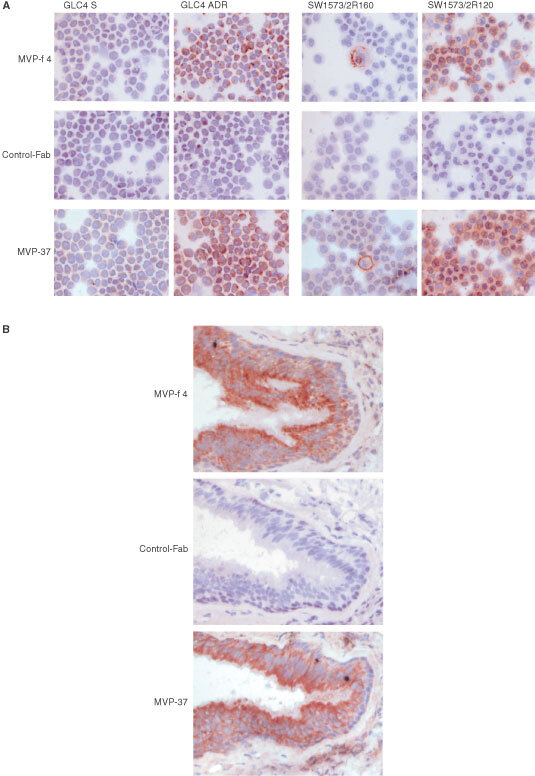
Staining results for MVP in a panel of cell lines with known presence or absence of MVP (A) and in frozen sections of normal human lung (B). Preparations were pretreated using the guanidine method (see Materials and methods) and stained with MVP-φ4, control Fab or MVP-37 murine Mab. Biotinylated rabbit-anti-mouse and HRP-labelled streptavidin were used as secondary reagents for the mouse Mab. For detection of the human Fabs, 9E10 Mab and HRP-labelled rabbit-anti-mouse serum was used. Colour development was with AEC/H2O2. Both the anti-MVP Fab MVP-φ4 and the murine Mab MVP-37 show, as expected, the presence of MVP in the GLC4 ADR cells, the SW1573/2R120 cells and in a characteristic low percentage of cells in the MDR1 P-gp positive SW1573/2R160 cells (A). Furthermore, MVP-φ4 and MVP-37 show the presence of MVP in the epithelial cells of the bronchi of the lung (B).
DISCUSSION
For the detection of MVP and transmembrane transporter molecules mediating MDR several conventionally produced murine Mabs have become available. The phage display method allows selection of human antibody fragments, which are expected to elicit less or no immune responses when administered in humans. Using this new technology various human antibodies have now been generated, and some of these have already entered phase III clinical applications (Holliger and Hoogenboom, 1998; Vaughan et al, 1998). Up till now, for none of the molecules associated with MDR these type of human antibodies have been generated. Only for MDR1 P-gp humanised versions of the monoclonal antibodies MRK16 (Hamada et al, 1990) and MRK17 (Ariyoshi et al, 1992) have been constructed. Also, the phage display method has been used successfully to identify peptide epitopes of MDR1 P-gp Mabs (Poloni et al, 1995a,b) and peptides mimicking the drug-binding activity of P-gp (Popkov et al, 1998). The present selection of a human anti-MVP reactive Fab by the phage display method is, therefore, the first report on truly human antibodies in this field.
During the selection steps of the anti-MVP Fab, successive selection rounds yielded increasing numbers of MVP-specific Fabs. However, BstNI fingerprint and sequence analyses showed that eventually only one type of Fab was isolated. The selection of just one type of Fab may have resulted from the way the selections were performed, causing loss of low affinity phages, e.g. due to low amounts of antigen for selection and high stringency conditions at the elution steps. Also, utilising recombinant MVP as the selection antigen, that may not have full native conformation, and the apparent presence of selection-dominant epitopes in most antigens (Hoogenboom et al, 1999) may have influenced the outcome of the selections. On the other hand, although the presently used library has been successfully applied for isolating Fabs against rare antigens and self antigens (de Haard et al, 1999), it cannot be excluded that these results still reflect library limitations.
In any case, the isolated MVP-φ4 Fab performed very well in several immunohistochemical and Western blot applications. The immunohistochemical results were optimal when the cytospins and tissues were pretreated with denaturing guanidine/HCl solution. These results, and the unreactivity of the Fab in immunoprecipitation experiments, suggest its reactivity with a linear epitope of the MVP molecule. Of note, both LRP-56 and LMR-5 Mabs are likely to react with more native epitopes, as judged from their good performance in immunoprecipitation experiments, in the absence of Western blot reactivity. Interestingly, BIAcore analyses showed that the MVP-φ4 Fab, as a monovalent fragment, has a relatively slow off-rate (1.3×10−3, s−1), as frequently observed with conventional antibodies generated from a secondary immune response. Indeed, the MVP-φ4 Fab off-rate was within the range observed for other (bivalent) murine anti-MVP Mabs.
The present identification of an MVP-specific Fab may allow for the construction of plasmids for functional knock-out purposes. The cDNA encoding the Fab can be transferred to an eukaryotic expression plasmid which may also provide a fusion between the Fab and an endoplasmatic reticulum retention signal. Transfection of this new construct into MVP-positive tumour cells then might lead to blockage of the MVP protein, e.g. due to binding of the Fab to the epitope during protein synthesis, and thus interfere with vault assembly and/or function. Recently, the expression of only the MVP molecule in Sf9 insect cells was shown to be sufficient for the assembly of vault-like particles (Stephen et al, 2001). Targeting this critical vault component, therefore, is very likely to result in the knock-out of functional vault particles. Analogous applications of ‘intrabodies’ have already been described for e.g. erbB-2 (Deshane et al, 1994), Ras (Lener et al, 2000), huntingtin (LeCerf et al, 2001), as well as the anti-P-gp directed C219 ScFv (Heike et al, 2001).
In conclusion, a human Fab fragment reactive to MVP, named MVP-φ4, was isolated by phage display technology. This Fab may become a valuable tool in studying the contribution of MVP and vaults to normal physiology and MDR.
Acknowledgments
This work was supported in part by Koningin Wilhelmina Fonds (KWF) grant EUR 98-1754 to EAC Wiemer and RJ Scheper and by the Netherlands Technology Foundation (STW), grant MGN55.3858, 805.17.753 to SHW Beiboer and HR Hoogenboom.
References
- AmbudkarSVDeySHrycynaCARamachandraMPastanIGottesmanMM1999Biochemical, cellular, and pharmacological aspects of the multidrug transporter Ann Rev Pharmacol Toxicol 39361398 [DOI] [PubMed] [Google Scholar]
- AriyoshiKHamadaHNaitoMHeikeYSeimiyaHMaezawaKTsuruoT1992Mouse-human chimeric antibody MH171 against the multidrug transporter P-glycoprotein Jpn J Cancer Res 83515521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BorstPEversRKoolMWijnholdsJ2000A family of drug transporters: the multidrug resistance-associated proteins J Natl Cancer Inst 9212951302 [DOI] [PubMed] [Google Scholar]
- ChanSGabraHHillFEvanGSikoraK1987A novel tumour marker related to the c-myc oncogene product Mol Cell Probes 17382 [DOI] [PubMed] [Google Scholar]
- ClacksonTHoogenboomHRGriffithsADWinterG1991Making antibody fragments using phage display libraries Nature 352624628 [DOI] [PubMed] [Google Scholar]
- de HaardHJvan NeerNReursAHuftonSERooversRCHenderikxPde BruineAPArendsJWHoogenboomHR1999A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies J Biol Chem 2741821818230 [DOI] [PubMed] [Google Scholar]
- DeshaneJLoechelFConryRMSiegalGPKingCRCurielDT1994Intracellular single-chain antibody directed against erbB2 down-regulates cell surface erbB2 and exhibits a selective anti-proliferative effect in erbB2 overexpressing cancer cell lines Gene Ther 1332337 [PubMed] [Google Scholar]
- DoyleLAYangWDAbruzzoLVKrogmannTGaoYMRishiAKRossDD1998A multidrug resistance transporter from human MCF-7 breast cancer cells (erratum in PNAS USA 1999; 96(5): 2569) Proc Natl Acad Sci USA 951566515670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FlensMJSchefferGLvan der ValkPBroxtermanHJEijdemsEWHuysmansACIzquierdoMAScheperRJ1997Identification of novel drug resistance-associated proteins by a panel of rat monoclonal antibodies Int J Cancer 73249257 [DOI] [PubMed] [Google Scholar]
- HamadaHMiuraKAriyoshiKHeikeYSatoSKameyamaKKurosawaYTsuruoT1990Mouse-human chimeric antibody against the multidrug transporter P-glycoprotein Cancer Res 5031673171 [PubMed] [Google Scholar]
- HeikeYKasonoKKunisakiCHamaSSaijoNTsuruoTKuntzDARoseDRCurielDT2001Overcoming multi-drug resistance using an intracellular anti-MDR1 sFv Int J Cancer 92115122 [PubMed] [Google Scholar]
- HigginsCF1992ABC transporters – from microorganisms to man Ann Rev Cell Biol 867113 [DOI] [PubMed] [Google Scholar]
- HolligerPHoogenboomH1998Antibodies come back from the brink Nat Biotechnol 1610151016 [DOI] [PubMed] [Google Scholar]
- HoogenboomHRde BruineAPHuftonSEHoetRMArendsJWRooversRC1998Antibody phage display technology and its applications Immunotechnology 4120 [DOI] [PubMed] [Google Scholar]
- HoogenboomHRGriffithsADJohnsonKSChiswellDHudsonPWinterG1991Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains Nucleic Acids Res 1941334137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HoogenboomHRLutgerinkJTPelsersMMRouschMJCooteJvan NeerNde BruineAVan NieuwenhovenFAGlatzJFArendsJW1999Selection-dominant and non-accessible epitopes on cell-surface receptors revealed by cell-panning with a large phage antibody library Eur J Biochem 260774784 [DOI] [PubMed] [Google Scholar]
- KickhoeferVARajavelKSSchefferGLDaltonWSScheperRJRomeLH1998Vaults are up-regulated in multidrug-resistant cancer cell lines J Biol Chem 27389718974 [DOI] [PubMed] [Google Scholar]
- KickhoeferVASivaACKedershaNLInmanEMRulandCStreuliMRomeLH1999bThe 193-kD vault protein, VPARP, is a novel poly (ADP-ribose) polymerase J Cell Biol 146917928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KickhoeferVAStephenAGHarringtonLRobinsonMORomeLH1999aVaults and telomerase share a common subunit, TEP1 J Biol Chem 2743271232717 [DOI] [PubMed] [Google Scholar]
- KitazonoMSumizawaTTakebayashiYChenZSFurukawaTNagayamaSTaniATakaoSAikouTAkiyamaS1999Multidrug resistance and the lung resistance-related protein in human colon carcinoma SW-620 cells J Natl Cancer Inst 9116471653 [DOI] [PubMed] [Google Scholar]
- KuiperCMBroxtermanHJBaasFSchuurhuisGJHaismaHJSchefferGLLankelmaJPinedoHM1990Drug transport variants without P-glycoprotein overexpression from a human squamous lung cancer cell line after selection with doxorubicin J Cell Pharmacol 13541 [Google Scholar]
- LaurencotCMSchefferGLScheperRJShoemakerRH1997Increased LRP mRNA expression is associated with the MDR phenotype in intrinsically resistant human cancer cell lines Int J Cancer 7210211026 [DOI] [PubMed] [Google Scholar]
- LeCerfJMShirleyTLZhuQKazantsevAAmersdorferPHousmanDEMesserAHustonJS2001Human single-chain Fv intrabodies counteract in situ huntingtin aggregation in cellular models of Huntington's disease Proc Natl Acad Sci USA 9847644769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LenerMHornIRCardinaleAMessinaSNielsenUBRybakSMHoogenboomHRCattaneoABioccaS2000Diverting a protein from its cellular location by intracellular antibodies. The case of p21Ras Eur J Biochem 26711961205 [DOI] [PubMed] [Google Scholar]
- MarksJDHoogenboomHRBonnertTPMcCaffertyJGriffithsADWinterG1991By-passing immunization. Human antibodies from V-gene libraries displayed on phage J Mol Biol 222581597 [DOI] [PubMed] [Google Scholar]
- PoloniFPalumboSCianfrigliaMFeliciF1995aSelection of phage-displayed peptides mimicking an extracellular epitope of human MDR1-P-glycoprotein Physiol Chem Physics Med NMR 27271280 [PubMed] [Google Scholar]
- PoloniFRomagnoliGCianfrigliaMFeliciF1995bIsolation of antigenic mimics of MDR1-P-glycoprotein by phage-displayed peptide libraries Int J Cancer 61727731 [DOI] [PubMed] [Google Scholar]
- PopkovMLussierIMedvedkineVEstevePOAlakhovVMandevilleR1998Multidrug-resistance drug-binding peptides generated by using a phage display library Eur J Biochem 251155163 [DOI] [PubMed] [Google Scholar]
- SchefferGLSchroeijersABIzquierdoMAWiemerEACScheperRJ2000Lung resistance-related protein/major vault protein and vaults in multidrug-resistant cancer Curr Opin Oncol 12550556 [DOI] [PubMed] [Google Scholar]
- SchefferGLWijngaardPLFlensMJIzquierdoMASlovakMLPinedoHMMeijerCJCleversHCScheperRJ1995The drug resistance-related protein LRP is the human major vault protein Nature Med 1578582 [DOI] [PubMed] [Google Scholar]
- ScheperRJBroxtermanHJSchefferGLKaaijkPDaltonWSvan HeijningenTHvan KalkenCKSlovakMLde VriesEGvan der ValkPMeijerCJPinedoHM1993Overexpression of a M(r) 110,000 vesicular protein in non-P-glycoprotein-mediated multidrug resistance Cancer Res 5314751479 [PubMed] [Google Scholar]
- SchneiderEPaulDIvyPCowanKH1999Multidrug resistance Cancer Chemother Biol Resp Modif 18152177 [PubMed] [Google Scholar]
- SchroeijersABSchefferGLReursAWPijnenborgACLMAbbondanzaCWiemerEACScheperRJ2001Detection of the Mr 110,000 lung resistance-related protein LRP/MVP with monoclonal antibodies J Histochem Cytochem 4913791386 [DOI] [PubMed] [Google Scholar]
- SchroeijersABSivaACSchefferGLde JongMCBolickSCDukersDFSlootstraJWMeloenRHWiemerEKickhoeferVARomeLHScheperRJ2000The Mr 193,000 vault protein is up-regulated in multidrug-resistant cancer cell lines Cancer Res 6011041110 [PubMed] [Google Scholar]
- StephenAGRaval-FernandesSHuynhTTorresMKickhoeferVARomeLH2001Assembly of vault-like particles in insect cells expressing only the major vault protein J Biol Chem 2762321723220 [DOI] [PubMed] [Google Scholar]
- VaughanTJOsbournJKTempestPR1998Human antibodies by design Nat Biotechnol 16535539 [DOI] [PubMed] [Google Scholar]
- WinterGGriffithsADHawkinsREHoogenboomHR1994Making antibodies by phage display technology Annu Rev Immunol 12433455 [DOI] [PubMed] [Google Scholar]
- ZamanGJFlensMJvan LeusdenMRde HaasMMulderHSLankelmaJPinedoHMScheperRJBaasFBroxtermanHJBorstP1994The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump Proc Natl Acad Sci USA 9188228826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZijlstraJGde VriesEGMulderNH1987Multifactorial drug resistance in an adriamycin-resistant human small cell lung carcinoma cell line Cancer Res 4717801784 [PubMed] [Google Scholar]


