Abstract
We conducted a case–control study to investigate the role of early infections in the aetiology of childhood acute leukaemias. The study included 280 incident cases (240 acute lymphoblastic leukaemia and 40 acute non-lymphoblastic leukaemia) and 288 hospital controls, frequency matched by age, gender, hospital, catchment area of the hospital and ethnic origin. Data were obtained from standardised face-to-face interviews of the mothers. The interviews included questions on early common infections, day-care attendance, breast-feeding, birth order and infantile diseases. Odds ratios were estimated using an unconditional regression model including the stratification variables, parental socio-economic status and perinatal characteristics. Birth order was not associated with childhood leukaemia (acute lymphoblastic or acute non-lymphoblastic). A statistically-significant inverse association was observed between childhood leukaemia and day-care attendance (odds ratio=0.6, 95% Confidence Interval=(0.4–1.0)), repeated early common infections (⩾4 per year before age two, odds ratio=0.6 (0.4–1.0)), surgical procedures for ear–nose–throat infections before age two (odds ratio=0.5 (0.2–1.0)) and prolonged breast-feeding (⩾6 months, odds ratio=0.5 (0.2–1.0)). In the multivariate model including day-care attendance, early common infections and breast-feeding, results concerning breast-feeding remained unchanged. A statistically significant interaction between day-care attendance and repeated early common infections was observed. When the interaction was taken into account, the simple effects of day-care and early common infections disappeared (odds ratio=1.1 (0.5–2.3) and odds ratio=0.8 (0.5–1.3), respectively) while the joint effect of day-care attendance and early common infections was negatively associated with childhood leukaemia (odds ratio=0.3 (0.1–0.8)). All the above associations were observed both for acute lymphoblastic leukaemia and acute non-lymphoblastic leukaemia. Our results support Greaves' hypothesis, even though they are not specific of common leukaemia.
British Journal of Cancer (2002) 86, 1064–1069. DOI: 10.1038/sj/bjc/6600091 www.bjcancer.com
© 2002 Cancer Research UK
Keywords: childhood leukaemia, risk factors, day-care, early infections, breast-feeding
Little is known about the aetiology of childhood acute leukaemia (AL), which is the most frequent childhood cancer world-wide (Doll, 1989; Ross et al, 1994). An infectious aetiology has been suggested for many years, particularly since specific viruses have been shown to be involved in leukaemia in animals (Essex, 1982). However, no specific virus has been found to explain childhood leukaemia. Kinlen postulated that childhood leukaemia occurs as a rare response to a specific infection(s) and increased by marked rural–urban population mixing (Kinlen, 1988, 1995; Kinlen et al, 1990; Kinlen and Petridou, 1995). Greaves hypothesised that common B-cell leukaemia, which is responsible for the incidence peak observed between ages 2 and 5 years, may result from a two-step process, with a first step occurring in utero (Greaves, 1988). Greaves suggested that the risk of childhood common B-cell leukaemia is increased by an immune proliferative stress. By contributing to the normal maturation of the immune system, early common infections or factors favouring infections in childhood would thus protect the child against leukaemia, while a situation of relative isolation would make the child more vulnerable (Greaves and Alexander, 1993; Greaves, 1997).
This article reports the results of a French case–control study designed to investigate the role of early common infections and factors influencing early common infections (day-care attendance, breast-feeding, and birth order) in childhood AL.
SUBJECTS AND METHODS
Subjects
A hospital-based case–control study was conducted in the hospitals of Lille, Lyon, Nancy and Paris (France). To be eligible, cases were required to be aged 15 years or less, reside in the hospital catchment area, and have a recent diagnosis of AL, i.e. diagnosis between January 1, 1995 and December 31, 1999. The hospital-based design of the study was chosen since case and control blood samples were required. Special care was therefore paid to selecting an appropriate control group. The controls were children hospitalised in the same hospital as the cases, mainly in orthopaedic and emergency departments, and residing in the catchment area of the hospital. Many different diagnostic categories were included in order to avoid selection biases in the event that a particular disease was related to the exposures of interest (Breslow and Day, 1980; Rothman and Greenland, 1998). However, children hospitalised for cancer or a major congenital malformation were not eligible for the study, since those diseases may share risk factors with leukaemia. Recruitment was frequency matched by age, gender, hospital, hospital catchment area and ethnic origin (Caucasian, North African, others). Of the mothers of the 282 cases and 291 controls who were eligible for interview during the interviewers' working hours, two cases and two control mothers refused to participate. We excluded one control child who was adopted. Thus, a total of 280 incident cases of AL confirmed by cytology, consisting of 240 cases of acute lymphoblastic leukaemia (ALL) and 40 cases of acute non-lymphoblastic leukaemia (ANLL), and 288 controls were included in the study.
Data collection
The mothers of the cases and controls were interviewed when the index child was in complete remission or in good condition (on average, 2 months post-diagnosis), using a standard questionnaire administered by trained medical interviewers. Interviews were performed in the hospitals under strictly similar conditions for the cases and controls. Neither the parents nor the interviewers were informed of the hypothesis underlying the study. Data relating to early infections and factors promoting infections included: birth order of the index child; interval to birth of the immediately elder sibling (intervals less than 2 and less than 5 years were examined); duration of breast-feeding; history of day-care attendance; history of early common infections; history of surgical procedures for early ear–nose–throat (ENT) infections; and infantile diseases. ‘Repeated early common infections’ was defined as four or more common infections per year before age 2. Surgical procedures for early ENT infections were defined as: adenoidectomy, tonsillectomy, tympanostomy tube insertion and tympanocentesis before age 2 years. The procedures were used as a surrogate for early, repeated, ENT infections.
Statistical analysis
All analyses were performed using the SAS computer software. Odds ratios (OR) were estimated using an unconditional logistic regression model including stratification variables, i.e. gender, age, ethnic origin and hospital. The socio-demographic characteristics (maternal educational level and parental socio-professional category) and perinatal characteristics (birth weight, length of pregnancy and number of pregnancies) were taken into account as potential confounders. The analyses of day-care attendance, early infections, breast-feeding and infantile diseases were conducted on the children aged over 2 years in order to be certain that early infections before age 2 would have already taken place in both the cases and controls. In the same way, multivariate analyses were conducted on the children aged over 2 years. Testing for interactions was systematically conducted. Two different final models were generated using two different variables as markers of early infections. In one model, ‘repeated infections before age 2’ and, in the other, ‘surgical procedures for ENT infections before age 2’ were used. In both, day-care and breast-feeding were included.
RESULTS
Most of the controls (88%) were recruited in an orthopaedic or emergency department (Table 1). Sixty per cent of the cases were 2–6 years old, vs 55% of the controls. The recruitment of controls in the age bracket 2–6 years (i.e. the childhood leukaemia incidence peak) was very difficult. Cases and controls were very similar with respect to gender, hospital, hospital catchment area, ethnic origin, maternal occupation at the time of interview, maternal educational level, parental socio-professional category and urban/rural residence status (Table 1). The cases and controls did not differ with respect to birth weight, length of pregnancy or number of pregnancies. However, reduced length of pregnancy and low birth weight were both, and independently, negatively related to prolonged breastfeeding (>6 months). Conversely, parity was positively related to prolonged breastfeeding.
Table 1. Sample description for the cases and controls.

No association between birth order and childhood leukaemia (ALL or ANLL) was observed (Table 2). The OR associated with a time interval to immediately elder sibling birth of less than 2 years was less than unity, but the association was far from statistical significance (OR=0.6, 95% Confidence Interval=(0.2–1.7)). The OR was close to unity when the interval to birth of the immediately elder sibling was less than 5 years (OR=0.8 (0.6–1.3)).
Table 2. Association between childhood acute leukaemia and birth order and siblings.

The results for early infections, day-care attendance and breast-feeding are shown in Table 3. A statistically-significant inverse association between day-care attendance and childhood AL (OR=0.6 (0.4–1.0)) was observed. The association was more pronounced for children having started day-care at age 6 months or less (OR=0.5 (0.3–1.0)) than for children having started day-care at age 13 months or more (OR=0.8 (0.3–1.8)). Nevertheless, the trend for age of starting day-care was not statistically significant. Repeated common infections before age 2 and surgical procedures for ENT infections before age 2 were statistically and negatively associated with childhood leukaemia (OR=0.6 (0.4–1.0) and OR=0.4 (0.2–1.0), respectively). Lastly, breast-feeding for at least 6 months was negatively associated with childhood leukaemia with an OR of 0.5 (0.3–1.1) and an OR of 0.5 (0.2–1.0) after adjustment for perinatal characteristics (birth weight, length of pregnancy, number of pregnancies).
Table 3. Association between childhood acute leukaemia and day care, early infections and breast-feeding in children older than 2 years.

The results of the joint analyses of early infection and breast-feeding are shown in Table 4. The model including day-care, repeated infections before age 2 and breast-feeding, showed a significant interaction between day-care attendance and common infections before age 2 (OR=0.3 (0.1–0.8)). The model including day-care, surgical procedures for ENT infections before age 2 and breast-feeding, did not show any interaction. For both models, the estimations were not altered by the mutual adjustments. Similar results were observed for ALL (common or not) and ANLL.
Table 4. Association between childhood acute leukaemia and multivariate analyses, in children older than 2 years.

The variables of interest were identically distributed over the different diagnostic categories in the control group. Moreover, the estimations of the above associations remained the same when the control group was restricted to the main diagnostic categories, i.e. injury or osteoarticular diseases. The OR were: OR=0.3 (0.1–0.8) and OR=0.3 (0.1–0.7), respectively, for the joint effect of day-care and repeated early common infections; OR=0.4 (0.2–1.1) and OR=0.2 (0.1–0.8) for ENT infections before age 2; and OR=0.5 (0.2–1.3) and OR=0.4 (0.1–1.2) for breast-feeding.
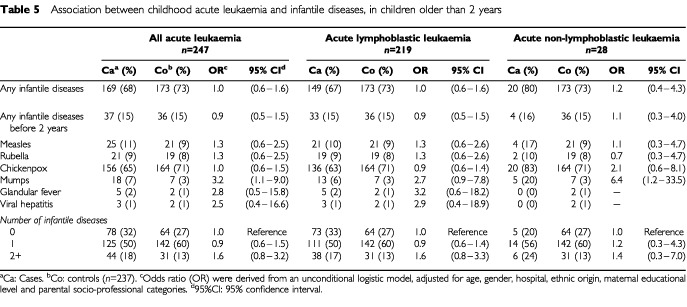
No association was found between measles, rubella or chickenpox and childhood leukaemia (Table 5). Elevated OR were observed for the association between childhood leukaemia and glandular fever and viral hepatitis, but based on very small numbers. A significant elevated OR was associated with mumps (OR=3.2 (1.1–9.0)).
Table 5. Association between childhood acute leukaemia and infantile diseases, in children older than 2 years.
DISCUSSION
Greaves suggested that early common infections in infancy and factors influencing early infections, such as birth order, older siblings, breast-feeding, and day-care, could have a protective effect against childhood AL (Greaves, 1988). A hospital-based case-control study was conducted in France to investigate the role of early infections in childhood AL. The hospital-based design of the study was chosen since case and control blood samples were required. Special care was therefore paid to selecting an appropriate control group. The reasons for which some case or control French-speaking mothers were not eligible for interview consisted in the non-availability or vacation of the interviewer, except for the mothers of two cases and two controls, who refused to participate. Controls were included from many diagnostic categories, none of those categories being related to the variables of interest. Our results were unchanged when the control group was restricted to each main diagnostic category. The cases and controls were very similar with respect to socio-demographic characteristics, i.e. maternal occupation at time of interview, maternal educational level, socio-professional categories and the rural/urban residential status.
Several previous studies on incident cases (Van Steensel-Moll et al, 1986; Petridou et al, 1997; Bener et al, 2001), and, in particular, several mortality studies (Stewart et al, 1958; MacMahon and Newill, 1962; Stark and Mantel, 1966) found that being the first-born increased the risk of, or mortality related to, childhood AL. We did not find such an association, in line with many other studies based on incident cases (Shaw et al, 1984; McKinney et al, 1987; Kaye et al, 1991; Savitz and Ananth, 1994; Cnattingius et al, 1995; Roman et al, 1996; Shu et al, 1999; Infante-Rivard et al, 2000; Neglia et al, 2000; Rosenbaum et al, 2000). An OR less than unity, but far from significance, was observed with respect to a time interval to birth of the immediately elder sibling of less than 2 years, as was reported by Kaye et al (1991), but not by Neglia et al (2000).
A statistically-significant inverse association was observed between day-care attendance and childhood AL, as has previously been reported by Petridou et al (1993) and Infante-Rivard et al (2000). That association was not observed in three other studies (Petridou et al, 1997; Neglia et al, 2000; Rosenbaum et al, 2000). It is noteworthy that, in Neglia's study (Neglia et al, 2000), children attended day-care more often than in our study (49% vs 27%), but started less often before age 1 than in our study (15% vs 21%). The statistically-significant interaction between day-care attendance and early common infections observed in our study suggests that infection in children attending day-care could differ in terms of frequency and/or type to those in other children. Diarrohea, upper respiratory tract infections and otitis have been shown to be more frequent in children attending day-care, compared to children not attending day-care (Haskins and Kotch, 1986; Wald et al, 1991; Reves et al, 1993). The statistically-significant inverse association between childhood AL and surgical procedures for ENT infection before age 2 is consistent with the results of a large study on ALL reported by Neglia et al (2000) in which the OR decreased as the number of episodes of otitis reported during the first year of life increases.
In our study, the surgical procedures for ENT infections before age 2 and day-care attendance among controls were significantly more frequent for urban residents than for rural residents. However, the cases and controls were similar with respect to urban/rural residential status, and our results remained unchanged when the analyses were restricted to urban children only.
Differential misclassifications such as under-declaration by the cases' mothers and/or over-declaration by the controls' mothers would seem minimal in the present study, due to the fact that the same standardised conditions were used to interview both the cases and the controls. Moreover, we obtained consistent results with respect to the mothers' declarations of their child's common infections before age 2 and the history of ENT surgery before age 2. The latter constitutes a less sensitive but more specific and more readily remembered surrogate of early infections. Similar results regarding the risk of childhood AL and early infections have already been reported in other studies. A negative association with infections during the first year of life was observed by Van Steensel-Moll et al (1986). McKinney et al (1999) observed a negative association with neonatal infections. Our results are also consistent with those of Neglia et al (2000). In contrast, two studies found no association with early infection (McKinney et al, 1987; Dockerty et al, 1999).
Breast-feeding for at least 6 months was statistically-significantly and negatively associated with childhood AL. That finding has also been reported in several recent case-control studies (Schüz et al, 1999; Shu et al, 1999; Smulevich et al, 1999; Infante-Rivard et al, 2000; Bener et al, 2001). Two studies found a reduced risk of childhood leukaemia, although the reductions were not significant (Davis et al, 1988; Dockerty et al, 1999). Other studies did not, however, evidence any association (Van Steensel-Moll et al, 1986; Magnani et al, 1988; McKinney et al, 1987; Golding et al, 1990; Shu et al, 1995; Petridou et al, 1997; Rosenbaum et al, 2000; Hardell and Dreifaldt, 2001). Except for two studies, one conducted in Shanghai (Shu et al, 1995) and the other in Sweden (Hardell and Dreifaldt, 2001), the duration of breast-feeding was not considered (Van Steensel-Moll et al, 1986; McKinney et al, 1987; Magnani et al, 1988; Golding et al, 1990; Petridou et al, 1997; Rosenbaum et al, 2000).
The usual infantile diseases – chickenpox, rubella and measles – were not associated with childhood AL. That finding is consistent with the results of recent studies (Dockerty et al, 1999; Schüz et al, 1999). McKinney et al (1987) observed an elevated OR (OR=4.1 (1.5–11.3)) between viral diseases comprising chickenpox, rubella, measles, mumps, viral meningitis, viral influenza and the risk of childhood leukaemia and lymphoma. In our study, an elevated and significant OR was also found for mumps (OR=3.2 (1.1–9.0)).
In conclusion, the main findings of the present study were the inverse relationships between childhood AL and early common infections, day-care and prolonged breast-feeding. These results are consistent with other publications and support Greaves' hypothesis, even though they are not specific to ALL.
Acknowledgments
We are grateful to Drs Diane Farkas, Kamila Kebaïli, Anne Lambilliotte, Dominique Steschenko, Martine Zagouri, and Naïma Belkacem, who conducted the interviews, and to Martine Valdes, Isabelle Jaussent, Laurence Mandereau, and Dominique Ridondelli for technical assistance. We also thank the heads of the departments who helped us to include their patients as controls: Professors Bensahel, Bérard, Carlioz, Deberigny, Felipe, Herbault, Lascombes, Pouliquen, and Rigault. We are grateful to Andrew Mullarky for his skilful revision of the manuscript. This work was supported by grants from Inserm, the French Ministère de l'Environnement et de l'Aménagement du Territoire, the Association pour la Recherche contre le Cancer, the Fondation de France, the Fondation Jeanne Liot, the Fondation Weisbrem-Benenson, the Ligue Contre le Cancer du Val de Marne and the Ligue Nationale Contre le Cancer.
References
- BenerADenicSGaladariS2001Longer breast-feeding and protection against childhood leukaemia and lymphomas Eur J Cancer 37234238 [DOI] [PubMed] [Google Scholar]
- BreslowNEDayNE1980Statistical Methods in Cancer Research, Vol. IInThe Analysis of Case-control studiesLyon: IARC Scientific Publications, N°32. IARC [PubMed] [Google Scholar]
- CnattingiusSZackMMEkbomAGunnarskogJKreugerALinetMAdamiHO1995Prenatal and Neonatal Risk Factors for Childhood Lymphatic Leukaemia J Natl Cancer Inst 87908914 [DOI] [PubMed] [Google Scholar]
- DavisMKSavitzDAGraubardBI1988Infant feeding and childhood cancer Lancet 2365368 [DOI] [PubMed] [Google Scholar]
- DockertyJDSkeggDCGHerbisonGPBecroftDMOLewisME1999Infections, vaccinations, and the risk of childhood leukemia Br J Cancer 8014831489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DollR1989The epidemiology of childhood leukaemia J R Statist Soc 152111 [Google Scholar]
- EssexME1982Feline leukemia: a naturally occuring cancer of infectious origin Epidemiol Rev 4189203 [DOI] [PubMed] [Google Scholar]
- GoldingJPatersonMKinlenLJ1990Factors associated with childhood cancer in a national cohort study Br J Cancer 62304308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GreavesMF1988Speculations on the cause of childhood acute lymphoblastic leukemia Leukemia 2120128 [PubMed] [Google Scholar]
- GreavesMFAlexanderFE1993An infectious etiology for common acute lymphoblastic leukemia in childhood? Leukemia 7349360 [PubMed] [Google Scholar]
- GreavesMF1997Aetiology of acute leukaemia Lancet 349344349 [DOI] [PubMed] [Google Scholar]
- HardellLDreifaldtAC2001Breast-feeding duration and the risk of malignant diseases in childhood in Sweden Eur J Clin Nutr 55179185 [DOI] [PubMed] [Google Scholar]
- HaskinsRKotchJ1986Day care and illness: evidence, cost, and public policy Pediatrics 77951982 [PubMed] [Google Scholar]
- Infante-RivardCFortierIOlsonE2000Markers of infection, breast-feeding and childhood acute lymphoblastic leukaemia Br J Cancer 8315591564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KayeSARobisonLLSmithsonWAGundersonPKingFLNegliaJP1991Maternal reproductive history and birth characteristics in childhood lymphoblastic leukaemia Cancer 6813511355 [DOI] [PubMed] [Google Scholar]
- KinlenLJ1988Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain Lancet ii13231326 [DOI] [PubMed] [Google Scholar]
- KinlenLJClarkeKHudsonC1990Evidence from population mixing in British new towns 1946-85 of an infective basis for childhood leukaemia Lancet 336577582 [DOI] [PubMed] [Google Scholar]
- KinlenLJ1995Epidemiological evidence for an infective basis in childhood leukemia Br J Cancer 7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KinlenLJPetridouE1995Childhood leukemia and rural population movements: Greece, Italy, and other countries Cancer Causes Control 6445450 [DOI] [PubMed] [Google Scholar]
- MacMahonBNewillVA1962Birth characteristics of children dying of malignant neoplasms J Natl Cancer Inst 28231244 [PubMed] [Google Scholar]
- MagnaniCPastoreGTerraciniB1988Infant feeding and childhood cancer Lancet 21136. [DOI] [PubMed] [Google Scholar]
- McKinneyPACartwrightRASaiuJMTMannJRStillerCADraperGJHartleyALHoptonPABirchJMWaterhouseJAHJohnstonHE1987The inter-regional epidemiological study of childhood cancer (IRESCC): a case-control study of aetiological factors for leukaemia and lymphoma Arch Dis Child 62279287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinneyPAJuszczakEFindlayESmithKThomsonCS1999Pre- and perinatal risk factors for childhood leukaemia and other malignancies: a Scottish case control study Br J Cancer 8018441851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NegliaJPLinetMSShuXOSeversonRKPotterJDMertensACWenWRobisonLL2000Patterns of infection and day care utilization and risk of childhood acute lymphoblastic leukaemia Br J Cancer 82234240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PetridouEKassimosDKalmantiMKosmidisHHaidasSFlytzaniVTongDTrichopoulosD1993Age of exposure to infections and risk of childhood leukaemia Br Med J 307774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PetridouETrichopoulosDKalapothakiVPourtsidisAKogevinasMKalmantiMKoliouskasDKosmidisHPanagiotouJPPiperopoulouFTzortzatouF1997The risk profile of childhood leukaemia in Greece: a nationwide case-control study Br J Cancer 7612411247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RevesRRMorrowALBartlettIIIAVCarusoCJPlumbRLLuBTPickeringLK1993Child Day Care Increases the Risk of Clinic Visits for Acute Diarrhea and Diarrhea Due to Rotavirus Am J Epidemiol 13797107 [DOI] [PubMed] [Google Scholar]
- RomanEAnsellPBullD1996Leukaemia and non-Hodgkin's lymphoma in children and young adults: are prenatal and neonatal factors important determinants of disease? Br J Cancer 76406415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RosenbaumPFBuckGMBrecherML2000Early Child-care and Preschool Experiences and the Risk of Childhood Acute Lymphoblastic Leukaemia Am J Epidemiol 15211361144 [DOI] [PubMed] [Google Scholar]
- RossJADaviesSMPotterJDRobisonLL1994Epidemiology of childhood leukaemia, with a focus on infants Epidemiol Rev 16243272 [DOI] [PubMed] [Google Scholar]
- RothmanKJGreenlandS1998Modern Epidemiology2nd edn,USA: Lippincott, Williams and Wilkins Publishers [Google Scholar]
- SavitzDAAnanthCV1994Birth characteristics of childhood cancer cases, controls, and their siblings Pediatr Hematol Oncol 11587599 [DOI] [PubMed] [Google Scholar]
- SchüzJKaletschUMeinertRKaatschPMichaelisJ1999Association of childhood leukaemia with factors related to the immune system Br J Cancer 80585590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShawGLaveyRJacksonRAustinD1984Association of childhood leukaemia with maternal age, birh weight, and paternal occupation: a case-control study Am J Epidemiol 119788795 [DOI] [PubMed] [Google Scholar]
- ShuXOClemensJZhengWYingDMJiBTJinF1995Infant Breastfeeding and the Risk of Childhood Lymphoma and Leukaemia Int J Epidemiol 242732 [DOI] [PubMed] [Google Scholar]
- ShuXOLinetMSSteinbuchMWenWQBuckleyJDNegliaJPPotterJDReamanGHRobisonLL1999Breast-feeding and risk of childhood acute leukaemia J Natl Cancer Inst 9117651772 [DOI] [PubMed] [Google Scholar]
- SmulevichVBSilionovaLGBelyakovaSV1999Parental occupation and others factors and cancer risk in children. Study methodology and non-occupational factors Int J Cancer 83712717 [DOI] [PubMed] [Google Scholar]
- StarkCRMantelN1966Effects of maternal age and birth order on the risk of mongolism and leukaemia J Natl Cancer Inst 37687698 [PubMed] [Google Scholar]
- StewartAWebbJHewittD1958A survey of childhood malignancies Br Med J 14951508 [DOI] [PMC free article] [PubMed]
- Van Steensel-MollHAValkenburgHAVan ZanenGE1986Childhood leukaemia and infectious diseases in the first year of life: a register-based case control study Am J Epidemiol 124590594 [DOI] [PubMed] [Google Scholar]
- WaldERGuerraNByersC1991Upper respiratory tract infections in young children: duration of and frequency complications Pediatrics 87129133 [PubMed] [Google Scholar]


