Abstract
Deregulated production of nitric oxide (NO) has been implicated in the development of certain human diseases, including cancer. We sought to assess the damaging potential of NO produced under long-term conditions through the development of a suitable model cell culture system. In this study, we report that when murine macrophage-like RAW264.7 cells were exposed continuously to bacterial lipopolysaccharide (LPS) or mouse recombinant interferon-γ (IFN-γ) over periods of 21–23 days, they continued to grow, but with doubling times 2 to 4 times, respectively, longer than the doubling time of unstimulated cells. Stimulated cells produced NO at rates of 30 to 70 nmol per million cells per day throughout the stimulation period. Within 24 hr after removal of stimulant, cells resumed exponential growth. Simultaneous exposure to LPS and IFN-γ resulted in decreased cell number, which persisted for 2 days after removal of the stimulants. Exponential growth was attained only after an additional 4 days. Addition of N-methyl-l-arginine (NMA), an NO synthase inhibitor, to the medium inhibited NO production by 90% of all stimulated cells, partially reduced doubling time of cells stimulated with LPS or IFN-γ, and partially increased viability and growth rates in those exposed to both LPS and IFN-γ. However, when incubated with LPS and IFN-γ at low densities both in the presence and in the absence of NMA, cells grew at a rate slower than that of unstimulated cells, with no cell death, and they resumed exponential growth 24 hr after removal of stimulants. Results from cell density experiments suggest that macrophages are protected from intracellularly generated NO; much of the NO damaging activity occurred outside of the producer cells. Collectively, results presented in this study suggest that the type of cellular toxicity observed in macrophages is markedly influenced by rate of exposure to NO: at low rates of exposure, cells exhibit slower growth; at higher rates, cells begin to die; at even higher rates, cells undergo growth arrest or die. The ability of RAW264.7 cells to produce NO over many cell generations makes the cell line a useful system for the study of other aspects of cellular damage, including genotoxicity, resulting from exposure to NO under long-term conditions.
Macrophages play a prominent role in host defense by inducing cellular damage in infectious agents and tumors (1, 2). One mechanism macrophages use to exert their cytotoxic and cytostatic effects on target cells is by releasing nitric oxide (NO) (reviewed in refs. 3–5). NO-mediated toxicity in target cells includes growth arrest through the formation of an iron–nitrosyl complex in mitochondrial enzymes, thereby inhibiting cellular respiration (6), energy depletion through deregulation of ATP metabolism (7), and programmed cell death (8–10). NO is a double-edged sword; excessive production of NO by macrophages is potentially toxic (11). Indeed, overproduction of NO has been implicated as a pathological factor in several forms of chronic human diseases, including arthritis (12), neurotoxicity (13), and cancer (14, 15).
Our laboratory has been interested in understanding the potential role of NO and reactive oxygen species in the development of chronic inflammation-associated cancer. Recent studies using an animal model system showed that NO produced in vivo was genotoxic in target tissues, and thus provide evidence in support of its involvement in the carcinogenic process (16). Since virtually all of the observations concerning the pathological role of overproduced NO have been made using animal models, the underlying mechanisms are poorly understood. Thus, it is essential to develop alternative experimental systems through which cellular damage induced by prolonged production of NO by macrophages can be studied in a well defined manner.
It is noteworthy that nearly all types of NO-mediated toxicity in target cells also occur in the producer macrophage cells (17–22). Thus, responses in macrophages and cocultured target cells as a result of NO production are related. For this reason, we examined the possibility of using macrophages as a model system for the study of NO-mediated cellular damage under long-term conditions. A number of macrophage cell lines have been found to produce NO at levels comparable to primary cells when appropriately stimulated (23). Commonly used stimuli for NO production by macrophages include the cytokine interferon-γ (IFN-γ) and bacterial lipopolysaccharide (LPS) (4). Among these cell lines, the mouse RAW264.7 cells have been shown to produce different levels of NO when stimulated with different combinations of IFN-γ and LPS (23, 24). Here we report that NO production rates and cell density can considerably influence the viability and growth of RAW264.7 cells. The results suggest that cellular responses in target cells, including macrophages, are largely governed by the rate at which cells are exposed to NO.
MATERIALS AND METHODS
Stimulation of RAW264.7 Cells with IFN-γ and/or LPS.
Cells of the mouse macrophage-like RAW264.7 line were obtained from the American Type Culture Collection (23) and then cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 1 mM l-glutamine (BioWhittaker). In experiments designed to compare cell growth under different stimulation conditions, 6 × 106 cells were cultured in 100-mm tissue culture plates (Falcon, polystyrene) in 10 ml of the above medium, to which was added one of the following agents to stimulate NO production: (i) 20 ng/ml Escherichia coli LPS (serotype O127:B8, Sigma); (ii) 20 units/ml recombinant mouse IFN-γ (Genzyme) plus 10 units/ml polymyxin B (PMB) sulfate (Sigma); or (iii) LPS plus IFN-γ without PMB. The composition of ii was designed to investigate the stimulatory effects of IFN-γ alone. PMB was added to inactivate any LPS that may have been an inadvertent contaminant of the IFN-γ preparations used. In parallel cultures, the NO synthase inhibitor N-monomethyl-l-arginine monoacetate (NMA) (Chem-Biochem Research, Salt Lake City, UT) was added to the medium to a concentration of 2 mM, to inhibit inducible NO synthase (iNOS) and thus identify effects attributable to NO. Every 24 hr, total NO production (nitrate plus nitrite content) was determined by analysis of medium by a previously described automated procedure (25). Cells were trypsinized and counted in a hemocytometer, then 6 × 106 cells from each plate were replated in fresh medium containing the appropriate agent(s). Cultures stimulated with agent iii generally contained less than 6 × 106 viable cells after the first day, in which case all surviving cells were replated. At the end of 3 days of continuous stimulation, cells were transferred to 10 ml of fresh medium containing 2 mM NMA (to block residual endogenous NO production) and incubated until an exponential growth rate was attained. During this recovery period, cell number was monitored and periodically adjusted to a density of 6 × 106 cells per plate to permit maximal growth. In experiments involving repeated stimulation, 6 × 106 cells were cultured in the presence of the agents for 3 days, then allowed to recover and resume exponential growth after their removal, as described above. This process was repeated twice. During each round of stimulation, total nitrite/nitrate level in the medium was measured.
In experiments involving continuous stimulation, 2 × 107 cells were incubated for 21–23 days in a 150-mm plate (Falcon, polystyrene) containing 35 ml of medium supplemented with either 100 ng/ml LPS or 20 units/ml IFN-γ plus 10 units/ml PMB, and also in the presence or absence of 2 mM NMA under both conditions. Nitrite concentration in the medium was estimated daily by using the Griess reagent (25), and medium was replaced when the concentration exceeded approximately 60 μM. Cell numbers were also determined periodically, and cell density was maintained at 1–2 × 107 cells per plate.
Stimulation of NO Production at Different Cell Densities: Effects on Growth and Viability.
One of our ultimate objectives is to study genotoxic responses in target cells cocultivated with NO-producing macrophages. For this purpose, hygromycin B-resistant RAW264.7 cells were established as follows. Cells were transfected with the P7hygro-9 vector containing the hygromycin resistance gene (a gift of Deborah Moshinsky, Massachusetts Institute of Technology) in a BTX (San Diego) electroporator, using parameters recommended by the manufacturer. Two days after electroporation, cells were replated in medium containing 500 μg/ml hygromycin B; after 2 weeks, surviving cells were maintained in medium containing 100 μg/ml hygromycin B. Integration of the hygromycin B-resistance gene had no detectable effect on doubling time or NO production in response to stimulation by IFN-γ and/or LPS (data not shown).
Hygromycin-resistant RAW264.7 cells were used in studies to evaluate the influence of cell density on growth and viability of cells stimulated with LPS and IFN-γ. To assess effects on cell growth, a total of 4 × 107 cells was divided into two or ten 150-mm plates, resulting in cell densities of 2 × 107 per plate and 4 × 106 per plate, respectively. Fresh medium was added to each plate to a final volume of 25 ml. Cells were stimulated with 20 units/ml IFN-γ plus 20 ng/ml LPS in the absence or presence of 2 mM NMA, as described above. After 48 hr of stimulation, the medium was replaced with medium containing only 2 mM NMA, and the cells were allowed to recover and resume growth. Unstimulated cells were cultured in parallel as negative controls.
To evaluate effects of cell density on viability, a total of 2 × 107, 4 × 106, 8 × 105, or 2 × 105 cells in 25 ml of medium was inoculated into one 150-mm plate, then stimulated with 20 units/ml IFN-γ plus 20 ng/ml LPS, in the absence or presence of 2 mM NMA. Unstimulated cells at each density were used as negative controls. After stimulation for 24 hr, cells were resuspended to dissociate siblings, and 24 hr later were trypsinized, pelleted, resuspended, and counted after staining with trypan blue to enumerate viable cells. Total nitrite plus nitrate content in medium from each density was determined as described above, except that medium from the two lowest densities was concentrated 10-fold prior to analysis.
RESULTS
Growth Patterns of RAW264.7 Cells Stimulated with IFN-γ and/or LPS.
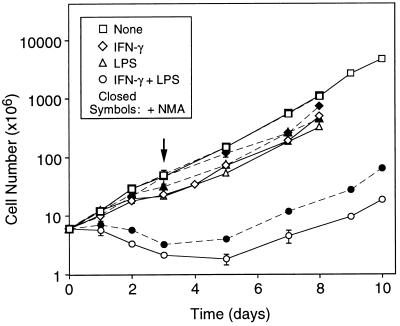
It has been commonly held that macrophages terminally differentiate and do not proliferate after activation (26). However, our early experiments indicated that under certain conditions RAW264.7 cells could survive stimulation by IFN-γ and LPS and eventually attain a growth rate equal to that of untreated cells. To characterize this response in further detail, cells were stimulated with LPS, IFN-γ plus PMB, or LPS and IFN-γ. After 3 days of stimulation, cells were transferred to medium lacking stimulating agents, but containing 2 mM NMA, added to block residual NO production. Results are summarized in Fig. 1. During the 3-day stimulation period, cells exposed to either LPS or IFN-γ alone grew more slowly than those cultured in the absence of either agent. After their removal, exponential growth was attained within 24 hr. In contrast, when cells were exposed to both agents simultaneously, cell numbers decreased not only during exposure, but also for 2 days after their removal. Exponential growth was attained only after an additional 4 days.
Figure 1.
Growth of RAW264.7 cells during and after three-day stimulation. Cells were incubated with no stimulus, LPS, IFN-γ and PMB, or LPS and IFN-γ, in the absence or presence of NMA. After 3 days of stimulation (arrow), the cells were incubated in growth medium supplemented with only NMA until they reached exponential growth. Each point is the mean ± SD of triplicate experiments.
To determine the extent to which these effects were associated specifically with NO production, cells were cultured in the presence of NMA, an NO synthase inhibitor, in addition to the stimulating agent(s). As shown in Fig. 1, NMA increased the growth rates of all stimulated cells, the effect being most marked on those cultured in the presence of both LPS and IFN-γ. Cell numbers increased by 40–60% throughout the stimulation period, and exponential growth was restored within 48 hr after removal of the agents. NMA had no effect on growth of unstimulated cells. These results confirmed that RAW264.7 cells stimulated by LPS or IFN-γ were capable of continued growth, and also indicated that multiple factors, including NO, contributed to cytostasis and cytotoxicity in stimulated cells.
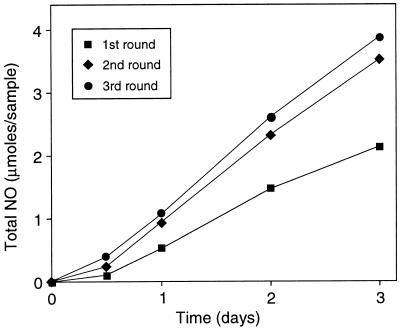
Because macrophages are known to be heterogeneous with respect to responsiveness to various stimuli (26), surviving cells in the above experiments could have included subpopulations insensitive to stimulation conditions used. We assessed this possibility by measurement of NO production after repeated stimulation. As shown in Fig. 2, cells produced NO in increasing amounts with each successive round of repeated stimulation, clearly demonstrating that cells surviving stimulation by LPS and IFN-γ were fully capable of NO production and were not representatives of a nonresponsive subpopulation.
Figure 2.
NO production by repeatedly stimulated RAW264.7 cells. Cells were stimulated with IFN-γ and LPS for 3 days with daily renewal of medium. NO production (total nitrite and nitrate) was measured at the times indicated. Following stimulation, cells were allowed to resume exponential growth. The protocol was repeated once and twice for second and third rounds, respectively. Each point is the mean ± SD of triplicate experiments (no SD exceeded 0.1 μmol per sample).
Growth and NO Production by RAW264.7 Cells During Protracted Continuous Stimulation.
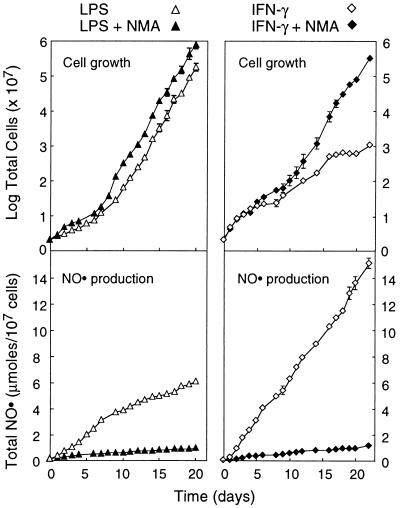
To extend the above observations, we subjected cells to long-term continuous stimulation by either LPS or IFN-γ, with the results summarized in Fig. 3. Cells stimulated with LPS for 21 days grew with an estimated doubling time of 35 hr and produced NO at a rate of 30 nmol per 106 cells per day. By comparison, the doubling time of untreated cells was 18–22 hr. Inclusion of 2 mM NMA in the medium blocked NO production by 90% but only slightly shortened the doubling time, to 30 hr. Cells stimulated with IFN-γ plus PMB for 23 days produced NO at a rate of 70 nmol per 106 cells per day, which was also effectively blocked by NMA. In this instance, NMA had a more pronounced effect on growth rate, in that cells grew with a doubling time of approximately 35 hr in its presence and 70 hr in its absence. Collectively, these results demonstrate that RAW264.7 cells stimulated continuously with LPS or IFN-γ are capable of growth and NO production over many cell generations. Additionally, it is of interest that cells cultured in the presence of IFN-γ continued to divide and produce NO even after 6 weeks of stimulation, whereas NO production by cells stimulated with LPS decreased gradually after 3 weeks (data not shown).
Figure 3.
Protracted production of NO by RAW264.7 cells continuously stimulated with LPS or IFN-γ. Cells were incubated with LPS alone or IFN-γ plus PMB, in the absence or presence of NMA. Cell numbers and NO production were measured at the times indicated. Each point is the mean ± SD of triplicate experiments.
Cell Density Effects on Growth and Viability in Macrophages Stimulated to Produce NO.
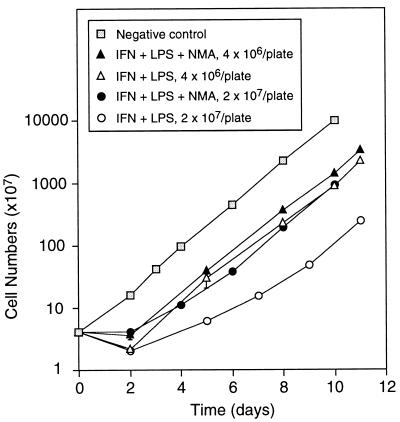
Our early experiments also indicated that density at which RAW264.7 cells were stimulated with IFN-γ and LPS had pronounced effects on the time required for resumption of growth after removal of stimulants from the medium. To further characterize this phenomenon, cells were stimulated at two different densities and their growth during and after stimulation was examined. As shown in Fig. 4, approximately 7 days were required for cells stimulated for 48 hr with LPS plus IFN-γ at a density of 2 × 107 cells per plate to resume exponential growth. This was achieved within only 2 days when NO production was inhibited by NMA. When cells were stimulated at a lower density, 4 × 106 cells per plate, cells resumed exponential growth within 2 days in both presence and absence of NMA. Thus, these results indicate that NO was cytostatic and that its cytostatic effect was cell-density dependent.
Figure 4.
Effect of cell density on growth of stimulated RAW264.7 cells. Cells plated at each indicated density were stimulated with LPS plus IFN-γ for 48 hr, in the absence or presence of NMA. Unstimulated cells were cultured in parallel as negative controls. After stimulation, cells were cultured in fresh medium supplemented with NMA, and cell numbers were determined at the times indicated. Each point is the mean ± SD of duplicate experiments.
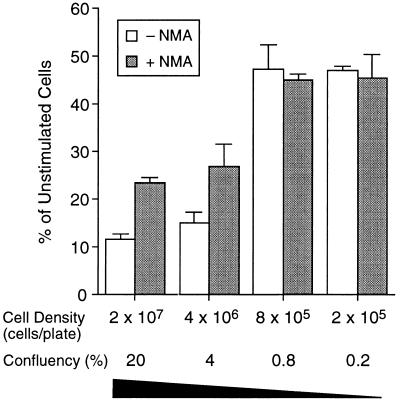
Although surviving cells resumed exponential growth at different rates, the numbers of viable cells remained the same at the two densities studied (Fig. 4). To examine whether density also affects viability, we stimulated cells at lower densities. Results shown in Fig. 5 demonstrate that viability was also affected by cell density during stimulation. At the two highest densities examined, viability of cells producing NO was reduced to approximately 50% of that of cells in which NO production was blocked by NMA, which was in agreement with results shown in Fig. 4. As cell density decreased, viability increased, to the extent that it was the same in the two lowest densities studied regardless of NMA. Furthermore, the numbers of cells stimulated at the two lowest densities increased by approximately 2-fold during the 48 hr of incubation with IFN-γ and LPS; these rates of growth were approximately half that of unstimulated cells. Total NO production was 120, 146, and 136 nmol per 106 cells at densities of 2 × 107, 4 × 106, and 8 × 105 cells per plate, respectively; addition of 2 mM NMA blocked NO production by 90% in all cases (data not shown). NO production in cells at the density of 2 × 105 per plate was below the limit of detection of the analytical procedure used. However, gene expression analysis showed that both iNOS and interleukin 1β mRNA could be detected in stimulated cells at this density (data not shown). Thus, NO production did not affect growth and viability of cells stimulated with IFN-γ and LPS at the two lowest densities, suggesting that macrophages as NO producer cells are protected from damage induced by intracellularly generated NO; much of the damaging activity of NO at higher densities could have been induced by NO from neighboring macrophage cells—i.e., extracellular NO.
Figure 5.
Effect of cell density on viability of stimulated RAW264.7 cells. Cells plated at the indicated densities were stimulated with IFN-γ plus LPS for 48 hr, in the absence or presence of NMA. Viable cells were counted after staining with trypan blue. Each data point is the mean ± SD of triplicate experiments.
DISCUSSION
Under the conditions used in these experiments, RAW264.7 macrophages cultured in the presence of either LPS or IFN-γ alone continued to grow, but at a rate somewhat slower than that of unstimulated cells. Within 24 hr after removal of stimulant, they resumed exponential growth. Simultaneous exposure to LPS and IFN-γ resulted in a decrease in cell number that persisted for 2 days after removal of the stimulants. Exponential growth was attained only after an additional 4 days. Blocking NO production with NMA partially restored growth rates of all stimulated cells, the effect being most marked on those exposed to both LPS and IFN-γ (Fig. 1). Thus, NO production in macrophages stimulated with IFN-γ and LPS was associated with both cytotoxicity and cytostasis. We ruled out the possibility that the surviving cells may have represented an unresponsive subpopulation by demonstrating that they produced NO when restimulated with IFN-γ and LPS (Fig. 2). Indeed, subsequent experiments showed that the capacity for NO production was undiminished in cells subjected to up to 7 successive rounds of stimulation (data not shown). However, cells surviving 9–10 rounds of repeated stimulation made less NO and at the same time had greater viability after treatment with IFN-γ and LPS. A subset of resistant cells cloned from this population retained the capacity for NO production when cultured in the presence of IFN-γ and LPS (unpublished results), in accord with recent findings of others (27, 28).
When cultured continuously in the presence of LPS or IFN-γ over periods of 21–23 days, macrophages continued to grow, but with doubling times 2 to 4 times, respectively, longer than that of unstimulated cells (Fig. 3). Stimulated cells produced NO at rates of 30–70 nmol per million cells per day throughout the stimulation period. Addition of NMA to the medium inhibited NO production by 90%, but it only partially reduced doubling times. Under these experimental conditions, no nonviable cells were detected by trypan blue staining, and all stimulated cells typically resumed exponential growth within 24 hr after removal of stimulant. Thus, NO production in macrophages stimulated with IFN-γ or LPS alone appeared to be growth inhibitory but not cytotoxic or cytostatic. Density at which cells were cultured during stimulation with LPS and IFN-γ markedly influenced their growth and viability. Cells plated at high density required approximately 4 times longer to resume exponential growth than those at low density (Fig. 4). Viability of cells plated at high density was reduced by approximately 40% compared with those at low density. The efficacy of NMA in preventing these effects, through inhibition of NO production, was similarly related to cell density (Fig. 5). Thus, at high density, NO production was both cytotoxic and cytostatic; as density decreased, NO production was cytotoxic but not cytostatic; as density further decreased, NO-mediated toxicity (cell death, cytostasis, and growth inhibition) disappeared. Similar density effects were also observed in cells stimulated with IFN-γ alone, in that at a higher density cytotoxicity was evident (data not shown). Collectively, these results indicate that NO-mediated damage (cell death, cytostasis, and growth inhibition) in macrophages was greatly influenced by the dose rate to which cells were exposed. At low rates of exposure, NO was growth inhibitory; at higher rates, it was cytotoxic; at even higher rates, it was both cytotoxic and cytostatic.
Mechanisms through which NO induces damage in exposed cells are presently not well characterized, but several pathways have been identified. NO is a relatively lipophilic, freely diffusible molecule readily released into the extracellular medium when produced by a macrophage cell (29). NO reacts with diffusion-limited kinetics with superoxide anion, generated concurrently by the same cell or produced by neighboring macrophages (30, 31). The product, peroxynitrite, is highly reactive and cytotoxic (ref. 30 and references therein). It has been estimated that RAW264.7 cells stimulated with IFN-γ and LPS generate twice as much NO as superoxide (31), thus providing abundant sources of peroxynitrite. Additionally, NO produced by cells can also undergo autoxidation to form the nitrosating agent N2O3, which can cause cell damage through its ability to induce deamination in cellular proteins and DNA (32). Small amounts of NO may diffuse through several cell diameters and eventually react with mitochondrial proteins in target cells, leading to cytostasis (29, 33). NO may also react with superoxide or hydrogen peroxide formed inside neighboring cells, thereby causing cell toxicity (11). Examination of NO-mediated damage under different levels of reactive oxygen species and oxygen tension, together with an understanding of the effects of NO exposure rate on macrophage metabolism, including lipid oxidation, mitochondrial enzyme activities, and stress protein expression, will help delineate the molecular basis through which NO causes cytostasis, death, or growth inhibition in exposed cells.
Inhibition of NO production by NMA did not restore the viability and growth of stimulated macrophages to levels comparable to those of unstimulated cells, suggesting the existence of other cytotoxic, cytostatic, and growth-inhibitory factors. As already noted above, RAW264.7 cells and other macrophages produce superoxide and hydrogen peroxide when stimulated with IFN-γ and LPS (31, 34, 35); macrophages defective in superoxide production survived stimulation by LPS (36). Therefore, it is possible that these reactive oxygen species themselves contribute to the cellular damage observed in our experiments. However, the extent of their contribution can be determined only by further study.
Frequent renewal of the culture medium increased cell viability, perhaps through maintenance of optimal medium pH and buffer capacity, as demonstrated by the ability of increased levels of sodium bicarbonate or Hepes in medium to increase cell viability (unpublished results). Several previous studies have shown that frequent renewal of macrophage culture medium results in production of higher amounts of NO per unit time and over longer periods of time (37–39). These effects have been attributed to one or more of the following: reexpression of iNOS mRNA (38); repletion of l-arginine; and prevention of post-translational inactivation of iNOS protein (39). Our results suggest that increased viability may be another contributing factor.
We observed that IFN-γ in the absence of LPS stimulated NO production by RAW264.7 cells, a finding that has been controversial. Others have reported that iNOS mRNA and nitrite/nitrate could be detected in mouse peritoneal macrophages incubated with IFN-γ alone (34, 40). However, other investigators found that RAW264.7 cells incubated with IFN-γ alone did not show iNOS gene expression or NO production (24). We also found that IFN-γ plus PMB was able to induce NO production in RAW264.7 cells, but the complete absence of LPS was not formally investigated. Thus, the possibility that our findings may have been caused by synergism between IFN-γ and trace levels of contaminating LPS (or other unidentified reagent contaminants) not inhibited by PMB cannot be ruled out.
As noted above, macrophages grown at low density during stimulation of NO production are capable of continued growth without evidence of cytotoxicity or cytostasis. Mechanisms underlying this capability of macrophages to protect themselves from damage by intracellularly generated NO have not been identified. Possible contributors include intracellular detoxifying agents such as glutathione (41) and/or heat shock protein (27). Alternatively, NO may be synthesized and delivered to the extracellular space in vesicle form, although efforts to identify membrane-bound NO synthase activity have yielded inconclusive results (42, 43).
Cultured macrophages provide a convenient and physiologically relevant model system for studying NO-mediated cellular damage, and indeed they develop a pattern of metabolic inhibition similar to that in tumor cells cocultured with them (8). However, results of such experiments must be interpreted cautiously, since no effort was made to differentiate the relative contributions of intracellular NO vs. extracellular NO (21, 44). In an in vivo setting, our results would imply that small numbers of NO-producing macrophages intermingled with other cell types in a tissue might be analogous to cells cultured at very low density, in that they would be spared from NO-mediated cytotoxicity. This suggestion is supported by results of several in vivo studies (29), including one from our laboratory, which showed that cells undergoing apoptosis were localized near macrophages expressing iNOS protein (10).
This study provides evidence suggesting that the nature of NO-mediated cellular damage in macrophages is governed by the rate at which cells are exposed to NO. It remains to be determined whether similar responses can be observed in other target cells when they are cocultured with NO-producing macrophages. Nonetheless, the establishment of conditions under which RAW264.7 cells can be stimulated to produce NO over prolonged periods makes this a useful model system for the study of cellular damage, including genotoxicity, in mammalian cells after long-term exposure to NO.
Acknowledgments
We thank Dr. Steven R. Tannenbaum for his valuable advice throughout the work, Dr. Teresa deRojas-Walker for providing RAW264.7 cells and assistance during the initial phase of the work, and Joseph Glogowski for nitrate and nitrite analysis. Financial support was provided by National Institutes of Health Grant CA26731 from the National Cancer Institute.
ABBREVIATIONS
- IFN-γ
interferon-γ
- LPS
lipopolysaccharide
- NMA
N-monomethyl-l-arginine
- PMB
polymyxin B
- iNOS
inducible NO synthase
References
- 1.Auger M J, Ross J A. In: The Macrophage: The Natural Immune System. Lewis C E, McGee J O’D, editors. New York: Oxford Univ. Press; 1992. pp. 1–74. [Google Scholar]
- 2.Adams D O, Hamilton T A. In: Inflammation: Basic Principles and Clinical Correlates. Gallin J I, Goldstein I M, Snyderman R, editors. New York: Raven; 1992. pp. 637–662. [Google Scholar]
- 3.Nathan C. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 4.Nathan C, Xie Q-w. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 5.Nathan C F, Hibbs J B., Jr Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 6.Drapier J-C, Hibbs J B., Jr J Clin Invest. 1986;78:790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo C, Zingarelli B, O’Connor M, Salzman A L. Proc Natl Acad Sci USA. 1996;93:1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui S, Reichner J S, Mateo R B, Albina J E. Cancer Res. 1994;54:2462–2467. [PubMed] [Google Scholar]
- 9.Mannick E E, Bravo L E, Zarama G, Reaple J L, Zhang X-J, Ruiz B, Fontham E T H, Mera R, Miller M J S, Correa P. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 10.Gal A, Tamir S, Kennedy L J, Tannenbaum S R, Wogan G N. Cancer Res. 1997;57:1823–1828. [PubMed] [Google Scholar]
- 11.Schmidt H H H W, Walter U. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 12.Connor J R, Manning P T, Settle S L, Moore W M, Jerome G M, Webber R K, Tjoeng F S, Currie M G. Eur J Pharmacol. 1995;273:15–24. doi: 10.1016/0014-2999(94)00672-t. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Dawson V L, Dawson T M, Snyder S H. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 14.Ohshima H, Bartsch H. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 15.Liu R H, Hotchkiss J H. Mutat Res. 1995;339:73–89. doi: 10.1016/0165-1110(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 16.Gal A, Wogan G N. Proc Natl Acad Sci USA. 1996;93:15102–15107. doi: 10.1073/pnas.93.26.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancaster J R, Jr, Hibbs J B., Jr Proc Natl Acad Sci USA. 1990;87:1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drapier J-C, Hibbs J B., Jr J Immunol. 1988;140:2829–2838. [PubMed] [Google Scholar]
- 19.Albina J E, Cui S, Mateo R B, Reichner J S. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- 20.Albina J E, Mastrofrancesco B. Am J Physiol. 1993;264:C1594–C1599. doi: 10.1152/ajpcell.1993.264.6.C1594. [DOI] [PubMed] [Google Scholar]
- 21.Meßmer U K, Lapetina E G, Brune B. Mol Pharmacol. 1995;47:757–765. [PubMed] [Google Scholar]
- 22.Zingarelli B, O’Connor M, Wong H, Salzman A L, Szabo C. J Immunol. 1996;156:350–358. [PubMed] [Google Scholar]
- 23.Stuehr D J, Marletta M A. Cancer Res. 1987;47:5590–5594. [PubMed] [Google Scholar]
- 24.Lorsbach R B, Murphy W J, Lowenstein C J, Snyder S H, Russell S W. J Biol Chem. 1993;268:1908–1913. [PubMed] [Google Scholar]
- 25.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 26.Adams D O, Hamilton T A. In: The Macrophage: The Natural Immune System. Lewis C E, McGee J O’D, editors. New York: Oxford Univ. Press; 1992. pp. 75–114. [Google Scholar]
- 27.Hirvonen M-R, Brune B, Lapetina E G. Biochem J. 1996;315:845–849. doi: 10.1042/bj3150845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brune B, Gotz C, Meßmer U K, Sandau K, Hirvonen M-R, Lapetina E G. J Biol Chem. 1997;272:7253–7258. doi: 10.1074/jbc.272.11.7253. [DOI] [PubMed] [Google Scholar]
- 29.Lancaster J R., Jr Proc Natl Acad Sci USA. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelm M, Dahmann R, Wink D, Feelisch M. J Biol Chem. 1997;272:9922–9932. doi: 10.1074/jbc.272.15.9922. [DOI] [PubMed] [Google Scholar]
- 31.Lewis R S, Tamir S, Tannenbaum S R, Deen W M. J Biol Chem. 1995;270:29350–29355. doi: 10.1074/jbc.270.49.29350. [DOI] [PubMed] [Google Scholar]
- 32.Tamir S, Tannenbaum S R. Biochim Biophys Acta. 1996;1288:F31–F36. doi: 10.1016/0304-419x(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 33.Stuehr D J, Nathan C F. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuehr D J, Marletta M A. J Immunol. 1987;139:518–525. [PubMed] [Google Scholar]
- 35.Cunha F Q, Assreuy J, Xu D, Charles I, Liew F Y, Moncada S. Eur J Immunol. 1993;23:1385–1388. doi: 10.1002/eji.1830230631. [DOI] [PubMed] [Google Scholar]
- 36.Vodovotz Y, Kwon N S, Pospischil M, Manning J, Paik J, Nathan C. J Immunol. 1994;152:4110–4117. [PubMed] [Google Scholar]
- 37.Ding A H, Nathan C, Stuehr D J. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 38.deRojas-Walker T, Tamir S, Ji H, Wishnok J S, Tannenbaum S R. Chem Res Toxicol. 1995;8:473–477. doi: 10.1021/tx00045a020. [DOI] [PubMed] [Google Scholar]
- 39.Amano F, Akamatsu Y. Infect Immun. 1991;59:2166–2174. doi: 10.1128/iai.59.6.2166-2174.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng W, Thiel B, Tannenbaum C S, Hamilton T A, Stuehr D J. J Immunol. 1993;151:322–329. [PubMed] [Google Scholar]
- 41.Luperchio S, Tamir S, Tannenbaum S R. Free Radical Biol Med. 1996;21:513–519. doi: 10.1016/0891-5849(96)00219-5. [DOI] [PubMed] [Google Scholar]
- 42.Forstermann U, Schmidt H H H W, Kohlhaas K L, Murad F. Eur J Pharmacol. 1992;225:161–165. doi: 10.1016/0922-4106(92)90096-e. [DOI] [PubMed] [Google Scholar]
- 43.Vodovotz Y, Russell D, Xie Q-w, Bogdan C, Nathan C. J Immunol. 1995;154:2914–2925. [PubMed] [Google Scholar]
- 44.Assreuy J, Cunha F Q, Liew F Y, Moncada S. Br J Pharmacol. 1993;108:833–837. doi: 10.1111/j.1476-5381.1993.tb12886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]