Abstract
Dose-escalation study was performed to evaluate the maximum tolerated dose, recommended dose and toxicity profile of weekly irinotecan with daily carboplatin and concurrent thoracic radiotherapy in patients with locally advanced non-small-cell lung cancer. Thirty-one previously untreated patients with unresectable stage III non-small-cell lung cancer were enrolled in this study. Patients received weekly irinotecan plus carboplatin (20 mg m−2 daily for 5 days a week) for 4 weeks and thoracic radiotherapy (60 Gy in 30 fractions). The irinotecan dose was escalated from 30 mg m−2 in increments of 10 mg m−2. Four irinotecan dose levels were given and 30 patients were assessable. Their median age was 62 years (range: 52–72 years), 28 had a performance status of 0–1 and two had a performance status of 2, 12 had stage IIIA disease and 18 had IIIB disease. There were 19 squamous cell carcinomas, 10 adenocarcinomas, and one large cell carcinoma. The dose-limiting toxicities were pneumonitis, esophagitis, thrombocytopenia and neutropenia. The maximum tolerated dose of irinotecan was 60 mg m−2, with two patients developing grade 4 pulmonary toxicity and one patient died of pneumonitis (grade 5). The recommended dose of irinotecan was 50 mg m−2. Other grade 3 or 4 toxicities were nausea and vomiting. Three patients achieved complete remission and 15 had partial remission, for an objective response rate of 60.0%. The median survival time was 14.9 months, and the 1- and 2-year survival rates were 51.6% and 34.2%, respectively. The study concluded that the major toxicity of this regimen was pneumonitis. This therapy may be active against unresectable non-small-cell lung cancer and a phase II study is warranted.
British Journal of Cancer (2002) 87, 258–263. doi:10.1038/sj.bjc.6600464 www.bjcancer.com
© 2002 Cancer Research UK
Keywords: non-small cell lung cancer, irinotecan, carboplatin, chemoradiotherapy
In patients with unresectable stage III non-small-cell lung cancer (NSCLC), two or more cycles of cisplatin-based chemotherapy, with or followed by radiation, has been proven to enhance survival (American Society of Clinical Oncology, 1997). Chemotherapy is appropriate for selected patients who have a good performance status. In general, chemotherapy is either given first followed by radiation, or is administered concurrently with radiation. Concurrent chemoradiotherapy regimens employ chemotherapy agents as radiosensitisers. Most studies that have shown a benefit for chemoradiotherapy have used cisplatin- or carboplatin-based combinations (Dillman et al, 1990; Le Chevalier et al, 1991; Jeremic et al, 1995), and both drugs are known to be radiosensitizers (Schaake-Koning et al, 1992; Jeremic et al, 1996). New active agents, such as paclitaxel, docetaxel, gemcitabine, vinorelbine and irinotecan, have been introduced and clinical trials of these agents for NSCLC have yielded promising data. These agents have been compared with each other in a phase III study performed in patients with advanced NSCLC, and several studies have suggested the radiosensitising properties of these new agents (Tishler et al, 1992; Leonard et al, 1996; Mcginn et al, 1996; Okishio et al, 1996). However, the phase I and II studies combining these agents with radiotherapy have mostly been preliminary (Choy et al, 1994; Greco et al, 1996; Gregor, 1997; Mauers et al, 1998; Herscher et al, 1998). Irinotecan has a mechanism of action targeting the nuclear enzyme topoisomerase I as radiosensitiser in vitro (Okishio et al, 1996). A response rate of 32% was observed in untreated patients with advanced NSCLC (Fukuoka et al, 1992) while a recent phase III study showed that irinotecan in combination with cisplatin achieved a significantly better survival compared with the combination of cisplatin and vindesine in patients with metastatic NSCLC (Fukuoka et al, 2000). We have already reported that a phase I/II study of weekly irinotecan with concurrent radiotherapy showed acceptable toxicity (esophagitis, diarrhea, and pneumonitis)(Takeda et al, 1999). Carboplatin has also been investigated as a radiosensitizer. Several studies (Groen et al, 1995; Kunitoh et al, 1997; Atagi et al, 2000) of concurrent daily carboplatin and radiotherapy have suggested that this combination is feasible and reasonably effective. Irinotecan and carboplatin have independently shown a synergistic effect with ionizing radiation in preclinical studies (Douple et al, 1985; Okishio et al, 1996). Based on these findings, we conducted a phase I trial of daily carboplatin and weekly irinotecan with concurrent thoracic radiotherapy for the treatment of locally advanced NSCLC in order to find the optimum dose of irinotecan and to estimate the antitumor activity and toxicity profile of this therapy.
MATERIALS AND METHODS
Patients selection
Patients were eligible for this study if they had histologically or cytologically documented and locally advanced stage III NSCLC that was deemed unresectable. Other eligibility requirements included an age of less than 75 years, an Eastern Cooperative Oncology Group performance status (PS) of 0 to 2, no previous chemotherapy or radiotherapy, ability to give written informed consent, as well as adequate pretreatment haematologic function (leukocyte count ⩾4 000 μl−1, haemoglobin ⩾9.5 g dl−1, and platelet count ⩾100 000 μl−1), renal function (a normal serum creatinine concentration), hepatic function (transaminases ⩽twice the normal range and serum bilirubin level ⩽1.5 mg dl−1), and pulmonary function (PaO2 ⩾70 Torr, %DLco >60%). Patients were excluded if they had contralateral hilar lymph node metastasis, a serious pre-existing disease, or a radiation field that exceeded half of one lung. Patient's informed consent and approval of the institutional ethics committee were mandatory for participation in the trial.
Treatment plan
Irinotecan was administered as a 90-min intravenous infusion once weekly, and carboplatin was given as a 30-min infusion (20 mg m−2) prior to thoracic radiotherapy daily for 5 days each week. Irinotecan and carboplatin were both administered for 4 weeks.
Thoracic radiotherapy started on day 1 and was given to a total dose of 60 Gy in 2.0 Gy fractions, which were delivered five times a week for 6 weeks using a linear accelerator (⩾4MV). The treatment volumes consisted of original and boost volumes irradiated sequentially. The initial large-field target volume consisted of the primary tumour, mediastinum, and involved hilar of supraclavicular nodes (total dose, 40 Gy), and boost dose of 20 Gy was delivered to a volume that consisted of the primary tumour and involved nodes. A combination of parallel-opposed anterior and posterior and oblique fields was used. The maximal spinal cord dose did not exceed 40 Gy. The target volume of the primary tumour included the complete extent of the visible primary tumour as defined radiographically (by computed tomography) with a minimum 1.5 cm and a maximum 2.5 cm margin around the mass.
The following therapy is optional. If the patient became operable as a result of tumour regression, surgery was done within 1 month of the completion of chemoradiotherapy. If the patient remained inoperable, two cycles of cisplatin with vindesine (cisplatin 80 mg m−2 day 1 and vindesine 3 mg m−2 on days 1, 8, 15) were given as systemic chemotherapy.
Dose escalation schedule
The starting dose of irinotecan was 30 mg m−2 and this was escalated by 10 mg m−2 increments in every three patients. There was no interpatient escalation. The next scheduled dose of irinotecan was omitted when grade 3 leukopenia, thrombocytopenia, or grade 2 diarrhea was observed.
Both thoracic radiotherapy and intravenous carboplatin were withheld if grade 3 leukopenia, neutropenia, thrombocytopenia, or grade 4 esophagitis was observed and restarted as soon as possible after recovery to grade 3 esophagitis and grade 2 haematological toxicity.
Dose-limiting toxicity
Dose-limiting toxicity was defined as grade 3 or 4 nonhaematologic toxicity, excluding nausea, vomiting, and alopecia, as neutropenic fever (grade 3 neutropenia and >38°C) or as grade 4 haematologic toxicity according to the WHO criteria (World Health Organization, 1979). If irinotecan was omitted two times or more due to any toxicity or radiotherapy and daily carboplatin was postponed for more than one week because of grade 3 haematological toxicity or grade 4 oesophagitis, we decided this was dose-limiting toxicity. If dose-limiting toxicity was observed in one or two out of three patients, an additional three patients were scheduled to be treated at the same dose level, and dose escalation could then continue if the toxicity was only observed in one or two out of six patients. If the dose-limiting toxicity was observed in all three patients or in more than three out of six patients, that dose was defined as the maximum tolerated dose. Recommended dose was defined the previous dose level.
Response and toxicity evaluation
Responses were evaluated according to the World Health Organization (WHO) criteria and toxicity was assessed prior to any further non-protocol therapy according to the WHO criteria (World Health Organization, 1979). Pulmonary toxicity was recorded as Grade 0–5 according to late Radiation Therapy Oncology Group (RTOG) criteria (Robert et al, 1999) as follows: 0, none; 1, asymptomatic or mild symptoms, slight radiographic appearances; 2, moderate symptomatic fibrosis or pneumonitis, low-grade fever, patchy radiographic appearances; 3, severe symptomatic fibrosis or pneumonitis, dense radiographic changes; 4, severe respiratory insufficiency, continuous oxygen, assisted ventilation; and 5, fatal. All reported responses and toxicities were confirmed by independent extramural review. Survival was measured from the initiation of chemoradiotherapy to death, and survival curves were estimated using the Kaplan–Meier method (Kaplan and Meier, 1958).
RESULTS
Patient characteristics
Between May 1996 and July 1998, 31 patients with histologically or cytologically confirmed stage III NSCLC were enrolled in this dose escalation study. Their clinical characteristics are summarised in Table 1. Four dose levels of irinotecan were administered (Table 2), and 30 patients were assessable for toxicity and efficacy. The remaining one patient who enrolled into irinotecan dose level of 50 mg m−2 was ineligible because brain metastasis was confirmed after enrollment. For these 30 patients, the median age was 62 years (range: 52–72 years). The performance status was 0–1 in 28 patients, while it was 2 in two patients. Twelve patients were in stage IIIA and 18 were in stage IIIB. Their tumours included 19 squamous cell carcinomas, 10 adenocarcinomas, and one large cell carcinoma.
Table 1. Patient characteristics.
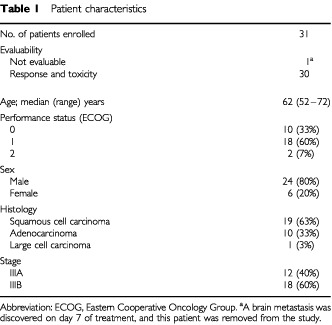
Table 2. Dose levels of irinotecan, dose actually delivered and dose intensity.
Actual doses of chemotherapy and radiotherapy
The planned individual drug doses, the actual delivered doses and dose intensity are listed in Table 2. Fourteen patients were treated with 30 mg m−2 of irinotecan. Although six patients should have been the maximum number in one step in our protocol, we added eight patients in first step to carry out this protocol safely because grade 4 pulmonary toxicity was observed in one patient, in the former study (Takeda et al, 1999) of weekly irinotecan combined with concurrent thoracic radiation therapy we experienced the treatment related death of pneumonitis and the Monitoring Committee of this protocol decided to add more patients in initial step. Administration of irinotecan was withheld due to neutropenia in three patients, disease progression in two patients, and diarrhea and localized erythema in one patient. Three patients did not complete the intravenous carboplatin schedule, one due to disease progression and the other due to a mistake about administration times. Dose intensities of irinotecan and carboplatin are listed in Table 2. The percentage of actually delivered dose-intensity of irinotecan and carboplatine was range from 91.1% to 100%. Twenty-five out of the 30 patients (83.3%) completed their radiotherapy as scheduled. The reason for not completing radiotherapy was disease progression in two patients and thrombocytopenia in one patient. Also, the first patient who received 60 mg m−2 of irinotecan suffered treatment-related death from pneumonitis and thrombocytopenia, so the other two patients treated at this dose level discontinued radiotherapy after 50 Gy.
Haematologic toxicity
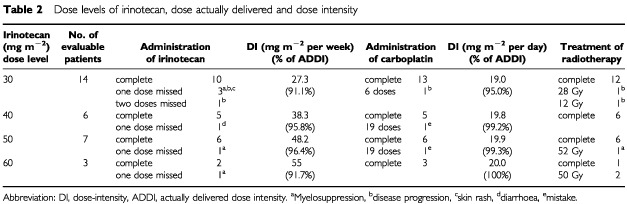
Thirty patients were assessable for haematologic toxicity, and the results summarised in Table 3. Haematologic toxicities were mild. The only grade 4 leukopenia and neutropenia were seen in one patient (grade 4 neutropenia) given 30 mg m−2 of irinotecan. G-CSF was administered to five of 14 patients on 30 mg m−2 of irinotecan, three of six on 40 mg m−2, four of seven on 50 mg m−2, and all three on 60 mg m−2 dose of irinotecan. Grade 4 thrombocytopenia occurred in two patients (one at the 50 mg m−2 and one at 60 mg m−2 doses of irinotecan) and this was dose-limiting toxicity. These two patients required platelet transfusions.
Table 3. Haematologic toxicity.
Nonhaematologic toxicity
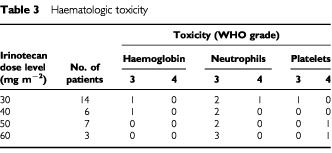
The nonhaematologic toxicities are summarised in Table 4. One patient suffered from grade 3 esophagitis at an irinotecan dose of 40 mg m−2, and two patients had grade 3 nausea with vomiting at 30 mg m−2 of irinotecan. No patient suffered from either grade 3 or 4 diarrhea. Grade 4 pneumonitis was observed in two patients treated with 60 mg m−2 of irinotecan, as well as in one patient each at both 30 mg m−2 and 40 mg m−2. Grade 5 pneumonitis was observed in one patient with 60 mg m−2 of irinotecan. Grade 4–5 pneumonitis was dose-limiting toxicity and was observed in all three patients at the 60 mg m−2 of irinotecan dose. Therefore we decided that this dose was defined as the maximum tolerated dose. Of these five patients who had grade 4–5 pneumonitis, all were treated with steroids and three required mechanical ventilation. Four patients eventually recovered, however one patient given 60 mg m−2 of irinotecan suffered treatment-related death. Pneumonitis seemed to be a principal toxicity of this combined modality.
Table 4. Nonhaematologic toxicity.
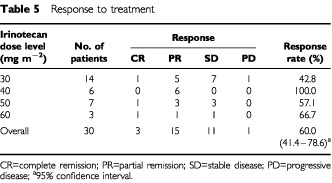
Response
The response to treatment is summarised in Table 5. Three patients achieved complete remission and 15 patients achieved partial remission, for an overall objective response rate of 60.0% (95% confidence interval 41.4–78.6%). Among the 18 responders, five patients underwent surgical resection of their residual disease and five received systemic chemotherapy with cisplatin and vindesine. Among the 11 patients with stable disease, four also received systemic chemotherapy.
Table 5. Response to treatment.
Survival and duration of response
The overall median survival time (MST) was 14.9 months, while the 1-year and 2-year survival rates were 51.6% and 34.2%, respectively. In the responding patients (i.e., those who achieved either complete or partial remission), the median duration of response was 11.0 months. In the patients who had either surgery or adjuvant chemotherapy, the MST was 21.9 months (range: 7.8 to 33.0 months) and 24.3 months (range: 5.4 to 32.4 months), respectively. In the other patients, the MST was 10.6 months (range: 1.1 to 36.9 months). The overall survival of all the patients is plotted in Figure 1.
Figure 1.
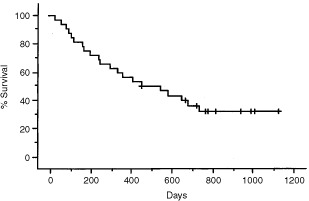
Overall survival. The estimated 1- and 2-year survival rate were 51.6 and 34.2%, and the median survival time was 14.9 months.
Pattern of failure
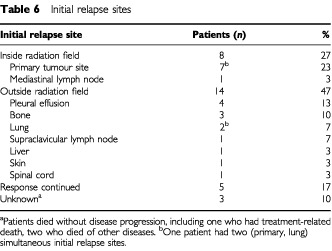
The sites of initial relapse are shown in Table 6. There were 22 sites of relapse in 29 patients who had partial remission or stable disease. The primary tumour inside the radiation field was the site of initial relapse in eight patients (seven without and one with distant metastasis), while distant metastasis was in ten patients and pleural effusion in four patients. Of five patients who underwent surgery, three patients had no relapse, one died of another disease, and one had pulmonary metastasis.
Table 6. Initial relapse sites.
DISCUSSION
Our present study showed that the combination of daily low-dose carboplatin and weekly irinotecan with concurrent thoracic radiotherapy is feasible. All three patients who received 60 mg m−2 of irinotecan developed grade 4–5 pneumonitis, although grade 4–5 pneumonitis was not observed at the 50 mg m−2 dose. In our former study of a phase I/II study (Takeda et al, 1999) of weekly irinotecan alone and concurrent thoracic radiotherapy in patients with stage III NSCLC, radiation therapy (2 Gy daily to a total dose of 60 Gy) was performed concurrently with administration of irinotecan done once weekly for 6 weeks. Twenty-seven patients were enrolled at three irinotecan dose levels (30, 45 and 60 mg m−2). In that phase I study, grade 4 pneumonitis occurred in one patient at a dose of 60 mg m−2, while in the phase II study using 45 mg m−2, one out of 10 patients developed severe toxicity (grade 4 pneumonitis plus grade 3 diarrhea) and died. In our study, the irinotecan administration period was reduced from 6 to 4 weeks because in our former study (Takeda et al, 1999) the number of patients increased who experienced the skip of the 5th and/or 6th administration of irinotecan. On the former study we added the daily carboplatin as another radiosensitiser.
Development of pulmonary toxicity is generally thought to be related to radiation dose, method of fractionation, and volume of the lung irradiated (Ginsberg et al, 1993). In patients receiving combined chemoradiotherapy, other confounding factors, such as the type of chemotherapeutic agent, also may play an important role in determining the risk of this toxicity. New chemotherapeutic agents, such as paclitaxel, have also been reported to show pulmonary toxicity (Choy et al, 1998). Therefore, the mechanism of pneumonitis seemed to be an interaction between all three parts of the treatment.
Recent studies suggest that analysis of the three-dimensional dose distribution gives useful data for the prediction of pulmonary toxicity (Martel et al, 1994; Marks et al, 1997; Graham, 1997). We could not calculate radiotherapy volume data since three-dimensional (3D) radiation therapy were not available with our study. So we calculated radiation portal size by two-dimensional treatment planning data. Radiation portal size was range from 105 m2 to 322 m2 (mean±SD; 179.7±48.0 m2). For five patients with grade 4 or 5 pulmonary toxicity, radiation field size was range from 168 m2 to 304 m2 (mean±SD; 208.8±54.4 m2). There was no significant relationship between radiation field size and pulmonary toxicity. It is very difficult to interpret the toxicity without more information about radiation volume data. This study thinks it is also worth reporting the premorbid lung function data, so we collected the individual data of pulmonary function tests (PFTs) before radiotherapy. Premorbid lung function data (including spirometry, volume measurements, and diffusion capacity) as follows (mean±SD): the per cent predicted vital capacity (%VC) 89.4±19.2%; the forced expiratory volume in 1 sec (FEV1) 1.88±0.58 L; the per cent predicted diffusion capacity to carbon monoxide (%DLCO) 90.1±21.7%. For five patients with grade 4 or 5 pulmonary toxicity, lung function data as follows (mean±SD): %VC 93.6±15.9%; FEV1.0 1.91±0.67L; %DLCO 78.0±23.5%. There was no relationship between PFT parameters and pulmonary toxicities. According these limited information, we suggest that pulmonary toxicity may be drug related rather than field size or baseline PFTs. In our study, radiation volume was not estimated, so we have to plan further study to reveal whether a dose and radiation volume are related to the occurrence of pulmonary toxicity.
The efficacy of combined-modality therapy for inoperable stage III NSCLC is reported to vary and the reason for this is unclear, although differences between the eligibility criteria used in various studies may account for the different outcomes (Mattson et al, 1988; Morton et al, 1991; Sause et al, 1995). In the present study, the maximum tolerated dose of irinotecan was 60 mg m−2. Pneumonitis, esophagitis, thrombocytopenia and neutropenia were the dose-limiting toxicities and pneumonitis was the principal toxicity of this regimen, whereas myelosuppression was mild. The overall response rate was 60.0%, while in patients given 50 and 40 mg m−2 of irinotecan, the response rate was 57.1 and 100%, respectively. At an irinotecan dose of 40 or 50 mg m−2, pneumonitis was manageable and haematologic toxicity was mild. Based on our findings we therefore decided that 50 mg m−2 of irinotecan was the recommended dose. Although these data are still preliminary, the median survival time was 14.9 months and the 1- and 2-year survival rates were 51.6 and 34.2%, respectively, which were reasonably good. When comparing these results with similar combined modality studies, the MST and survival rates are most encouraging. And the MST of 21.9 months and 24.3 months in patients who underwent surgery or had adjuvant chemotherapy was better than that in patients who had no additional treatment. It is because that optional treatment was done in responding patients or stable patients. It should be discussed whether in responding patients additional treatment is necessary.
In conclusion, irinotecan combined with daily carboplatin for 4 weeks and concurrent thoracic radiotherapy appears to be feasible and improve the survival of patients with unresectable locally advanced NSCLC. MTD and recommended dose of irinotecan were 60 mg m−2 and 50 mg m−2, respectively. Principal toxicity of this combined modality was pneumonitis. A phase II study of this combination is warranted.
References
- American Society of Clinical Oncology1997Clinical practice guidelines for the treatment of unresectable non- small-cell lung cancer. Adopted on May 16, 1997 J Clin Oncol 1529963018 [DOI] [PubMed] [Google Scholar]
- AtagiSKawaharaMOgawaraMMatsuiKMasudaNKudohSNegoroSFuruseK2000Phase II trial of daily low-dose carboplatin and thoracic radiotherapy in elderly patients with locally advanced non-small cell lung cancer Jpn J Clin Oncol 305964 [DOI] [PubMed] [Google Scholar]
- ChoyHAkerleyWSafranHClarkJRegeVPapaAGlantzMPuthawalaYSoderbergCLeoneL1994Phase I trial of outpatient weekly paclitaxel and concurrent radiation therapy for advanced non-small cell lung cancer J Clin Oncol 1226822686 [DOI] [PubMed] [Google Scholar]
- ChoyHSafranHAkerleyWGrazianoSLBogartJAColeBF1998Phase II trial of weekly paclitaxel and concurrent radiation therapy for locally advanced non-small cell lung cancer Clin Cancer Res 419311936 [PubMed] [Google Scholar]
- DillmanROSeagrenSLPropertKJGuerraJEatonWLPerryMCCareyRWFreiIIIEFGreenMR1990A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer N Engl J Med 323940945 [DOI] [PubMed] [Google Scholar]
- DoupleEBRichmondRCO'HaraJACoughlinCT1985Carboplatin as potentiator of radiation therapy Cancer Treat Rev 12111114 [DOI] [PubMed] [Google Scholar]
- FukuokaMNiitaniHSuzukiAMotomiyaMHasegawaKNishiwakiYKuriyamaTAriyoshiYNegoroSMasudaN1992A phase II study of CPT-11, a new derivative of camptothecin, for previously untreated non-small-cell lung cancer J Clin Oncol 101620 [DOI] [PubMed] [Google Scholar]
- FukuokaMNagaoKOhashiYNiitaniH2000Impact of irinotecan (CPT-11) and cisplatin (CDDP) on survival in previously untreated metastatic non-small cell lung cancer (NSCLC) Proc Am Soc Clin Oncol 19495a [Google Scholar]
- GinsbergRJKrisMGArmstrongJG1993Cancer of the lung: Non-small cell lung cancerInCancer: Principles and Practice of Oncology4th edn.,DeVita VT, Hellman S, Rosenberg S (eds)pp676723Philadelphia: Lippincott [Google Scholar]
- GrahamMV1997Predicting radiation response Int J Radiat Oncol Biol Phys 39561562 [DOI] [PubMed] [Google Scholar]
- GrecoFAStroupSLGrayJRHainsworthJD1996Paclitaxel in combination chemotherapy with radiotherapy in patients with unresectable stage III non-small cell lung cancer J Clin Oncol 1416421648 [DOI] [PubMed] [Google Scholar]
- GregorA1997Gemcitabine plus radiotherapy for non-small cell lung cancer Semin Oncol 243941 [PubMed] [Google Scholar]
- GroenHJvan der LeestAHde VriesEG1995Continuous carboplatin infusion during 6 weeks' radiotherapy in locally inoperable non-small-cell lung cancer Br J Cancer 72992997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HerscherLLHahnSMKroogGPassHTemeckBGoldspielBCookJMitchellJBLiebmannJ1998Phase I study of paclitaxel as radiation sensitizer in the treatment of mesothelioma and non-small-cell lung cancer J Clin Oncol 16635641 [DOI] [PubMed] [Google Scholar]
- JeremicBShibamotoYAcimovicLDjuricL1995Randomized trial of hyperfractionated radiation therapy with or without concurrent chemotherapy for stage III non-small-cell lung cancer J Clin Oncol 13452458 [DOI] [PubMed] [Google Scholar]
- JeremicBShibamotoYAcimovicLMilisavljevicS1996Hyperfractionated radiation therapy with or without concurrent low-dose daily carboplatin/etoposide for stage III non-small-cell lung cancer: a randomized study J Clin Oncol 1410651070 [DOI] [PubMed] [Google Scholar]
- KaplanELMeierP1958Nonparametric estimation from incomplete observations J Am Stat Assoc 53457481 [Google Scholar]
- KunitohHWatanabeKNagatomoAOkamotoHKimbaraK1997Concurrent daily carboplatin and accelerated hyperfractionated thoracic radiotherapy in locally advanced non-small cell lung cancer Int J Radiat Oncol Biol Phys 1103109 [DOI] [PubMed] [Google Scholar]
- Le ChevalierTArriagadaRQuoixERuffiePMartinMTarayreMLacombe-TerrierMJDouillardJYLaplancheA1991Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in unresectable non-small cell lung cancer: first analysis of a randomized trial in 353 patients J Natl Cancer Inst 83417423 [DOI] [PubMed] [Google Scholar]
- LeonardCEChanDCChouTCKumarRBunnPA1996Paclitaxel enhances in vitro radiosensitivity of squamous carcinoma cell lines of the head and neck Cancer Res 5651985204 [PubMed] [Google Scholar]
- MarksLBMunleyMTBentelGCZhouSMHollisDScarfoneCSibleyGSKongFMJirtleRJaszczakRColemanRETapsonVAnscherM1997Physical and biological predictors of changes in whole-lung function following thoracic irradiation Int J Radiat Oncol Biol Phys 39563570 [DOI] [PubMed] [Google Scholar]
- MartelMKTen HakenRKHazukaMBTurrisiATFraassBALichterAS1994Dose-volume histogram and 3-D treatment planning evaluation of patients with pneumonitis Int J Radiat Oncol Biol Phys 28575581 [DOI] [PubMed] [Google Scholar]
- MattsonKHolstiLRHolstiPJakobssonMKajantiMLiippoKMantylaMNiitamo-KorhonenSNikkanenVNordmanE1988Inoperable non-small-cell lung cancer: radiation with or without chemotherapy Eur J Cancer Clin Oncol 24477482 [DOI] [PubMed] [Google Scholar]
- MauersAMMastersGHarafDJHoffmanPCWatsonSMGolombHMVokesEE1998Phase I study of docetaxel with concomitant thoracic radiation therapy J Clin Oncol 16159164 [DOI] [PubMed] [Google Scholar]
- McGinnCJSherwachDSLawrenceTS1996Radiosensitizing nucleosides J Natl Cancer Inst 8811931203 [DOI] [PubMed] [Google Scholar]
- MortonRFJettJRMcGinnisWLEafleJDTherneauTMKrookJEElliottTEMailliardJANelimarkRAMaksymiukAW1991Thoracic radiation therapy alone compared with combined chemoradiotherapy for locally unresectable non-small cell lung cancer: a randomized, phase III trial Ann Intern Med 115681686 [DOI] [PubMed] [Google Scholar]
- OkishioKKudohSKuriharaNHirataKYoshikawaJ1996Irinotecan (CPT-11) enhances the radiosensitivity of lung cancer cells in vitro Cell Pharmacol 3247252 [Google Scholar]
- RobertFChildsHASpencerSAReddenDTHawkinsMM1999Phase I/IIa Study of Concurrent Paclitaxel and Cisplatin With Radiation Therapy in Locally Advanced Non-Small Cell Lung Cancer: Analysis of Early and Late Pulmonary Morbidity Semin Radiat Oncol 2Suppl 1136147 [PubMed] [Google Scholar]
- SauseWTScottCTaylorSJohnsonDLivingstonRKomakiREmamiBCurranWJByhardtRWTurrisiAT1995Radiation Therapy Oncology Group (RTOG) 88-08 and Eastern Cooperative Oncology Group (ECOG) 4558: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer J Natl Cancer Inst 87198205 [DOI] [PubMed] [Google Scholar]
- Schaake-KoningCvan den VogaertWDalesioOFestenJHoogenhoutJvan HouttePKirkpatrickAKoolenMMaatBNijsA1992Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer N Engl J Med 326524530 [DOI] [PubMed] [Google Scholar]
- TakedaKNegoroSKudohSOkishioKMasudaNTakadaMTanakaMNakajimaTTadaTFukuokaM1999Phase I/II study of weekly irinotecan and concurrent radiation therapy for locally advanced non-small cell lung cancer Br J Cancer 7914621467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TishlerRBGeardCRHallEJSchiffPB1992Taxol sensitizes human astrocytoma cells to radiation Cancer Res 5234953497 [PubMed] [Google Scholar]
- World Health Organization1979WHO Handbook for Reporting Results of Cancer TreatmentWHO Publication No.48. Geneva, Switzerland: World Health Organization [Google Scholar]


