Abstract
N-acetylgalactosaminyl transferase-3 (GalNAc-T3) is an enzyme involved in the initial glycosylation of mucin-type O-linked proteins. In the present study, we used immunohistochemistry to examine GalNAc-T3 expression in 215 surgically resected non-small cell lung cancers. We analysed the biological and clinical importance of GalNAc-T3 expression, especially with regard to its potential as a prognostic factor. We found that normal bronchial epithelial cells, bronchial gland cells, and alveolar pneumocytes showed cytoplasmic immunostaining for GalNAc-T3. Low expression of GalNAc-T3, observed in 93 of 215 tumours (43.4%), was found more frequently in tumours from smokers than those from nonsmokers (P=0.001), in squamous cell carcinomas than nonsquamous cell carcinomas (P<0.0001), and in moderately and poorly differentiated tumours than well differentiated tumours (P=0.0002). Multivariate logistic regression analysis showed that an association of low GalNAc-T3 expression with squamous cell carcinomas was the only one significant relationship of GalNAc-T3 expression with various factors (P<0.0001). Moreover, tumours losing GalNAc-T3 expression had a significantly higher Ki-67 labelling index than tumours retaining GalNAc-T3 expression (P=0.0003). Patients with low GalNAc-T3 expression survived a significantly shorter time than patients with high GalNAc-T3 expression in 103 pStage I non-small cell lung cancers (5-year survival rates, 58% and 78%, respectively; P=0.02 by log-rank test) as well as in 61 pStage I nonsquamous cell carcinomas (5-year survival rates, 63% and 85%, respectively; P=0.03). Low GalNAc-T3 expression was an unfavourable prognostic factor in pStage I non-small cell lung cancers (hazards ratio, 2.04; P=0.03), and in pStage I nonsquamous cell carcinomas (hazards ratio, 2.70; P=0.03). These results suggest that GalNAc-T3 is a new marker of non-small cell lung cancers with specificity for histology and prognosis.
British Journal of Cancer (2002) 87, 751–755. doi:10.1038/sj.bjc.6600536 www.bjcancer.com
© 2002 Cancer Research UK
Keywords: GalNAc-T3, lung cancer, adenocarcinoma, Ki-67, prognosis
Lung cancer is one of the leading causes of cancer death worldwide. Although the management and treatment of non-small cell lung cancers (NSCLCs) have improved, there is no evidence that therapeutic advances have resulted in a marked increase of survival rates. The overall 5-year survival rate remains at less than 15% (Ginsberg et al, 2001). It is not fully understood why patients with comparable stages of NSCLC may have different clinical courses and respond differently to similar treatments. A more sophisticated understanding of the pathogenesis and biology of these tumours (Hanahan and Weinberg, 2000; Sekido et al, 2001) could provide useful information for predicting clinical outcome, individualising treatment (Harpole et al, 1995; Strauss et al, 1995; Kwiatkowski et al, 1998; Dosaka-Akita et al, 2001), and identifying molecular targets of the treatment (Gibbs, 2000).
Oligosaccharides on glycoproteins are altered in tumorigenesis. These oligosaccharides often play a role in the regulation of the biological characteristics of tumours in terms of invasion and metastatic potential (Hakomori, 1989). Each oligosaccharide is synthesised by a specific glycosyltransferase (Varki, 1993). The initial glycosylation of mucin-type O-linked proteins is catalysed by one of the UDP-N-acetyl-α-D-Galactosamine: polypeptide N-acetylgalactosaminyl transferases (GalNAc-transferase family of enzymes) (Hagen et al, 1993; Homa et al, 1993; Wandall et al, 1997;). Three distinct human GalNAc-transferases, GalNAc-T1, GalNAc-T2, and GalNAc-T3, have been characterised (White et al, 1995; Bennett et al, 1996; Wandall et al, 1997). Recently another 3 homologue enzymes, GalNAc-T4, GalNAc-T5, and GalNAc-T6, have been identified (Bennett et al, 1996, 1998, 1999; Ten Hagen et al, 1998). Compared with the expression of GalNAc-T1 and GalNAc-T2, the expression of GalNAc-T3 is highly tissue specific. GalNAc-T3 mRNA has been detected in organs that contain secretory epithelial glands (Hagen et al, 1993; Homa et al, 1993; White et al, 1995; Bennett et al, 1996). It is hypothesised that the differential expression of GalNAc-T3 may affect the specialised functions of glycoproteins produced by normal and malignant cells, and that it is also associated with biological properties of normal and malignant cells (Sutherlin et al, 1997). However, GalNAc-T3 expression has not been previously examined in human lung cancers, or in human bronchial epithelial cells and alveolar pneumocytes, from which lung cancers develop.
In the present study, GalNAc-T3 expression was examined by immunohistochemistry in surgically resected NSCLCs. We analysed the biological and clinical importance of GalNAc-T3 expression, especially with regard to its potential as a prognostic factor.
MATERIALS AND METHODS
Tumour specimens and survival data
Primary tumour specimens from 215 NSCLCs were consecutively obtained by surgery from the Hokkaido University Medical Hospital during 1976 and 1994. The patients with NSCLCs consisted of 142 men and 73 women. The histologic classification of the tumour specimens was based on World Health Organization criteria (World Health Organization, 1982). The tumour specimens included 87 squamous cell carcinomas, 110 adenocarcinomas, nine large cell carcinomas, and eight adenosquamous cell carcinomas. Nonsquamous cell carcinoma included adenocarcinoma, large cell carcinoma and adenosquamous cell carcinoma. There were 119 Stage I, 18 Stage II, 70 Stage IIIa, one Stage IIIb, and seven Stage IV tumours. The postsurgical pathologic TNM stage (pTNM) was determined according to the guidelines of the American Joint Committee on Cancer (American Joint Committee on Cancer, 1992). Of the 119 patients with pStage I tumours resected with curative intent, survival was analysed for the 103 patients who met the following criteria: (1) survived for more than 3 months after surgery; (2) did not die of causes other than lung cancer within 5 years after surgery; and (3) were followed for more than 3 years after surgery (for patients who remained alive). Sixteen patients did not meet the above criteria (four died within 3 months after surgery, five died of causes other than lung cancer within 5 years, and seven had no survival records after surgery) were excluded from the survival analysis. Of the 103 patients for whom survival was analysed, 54 patients had died of cancer, including 27 with squamous cell carcinomas, 22 with adenocarcinomas, three with large cell carcinomas and two with adenosquamous cell carcinomas. The Karnofsky performance status was 90% or greater in all these 103 patients. This study was approved by the Medical Ethical Committees of Hokkaido University School of Medicine. Because all patients were coded, they could not be individually identified.
Construction of the plasmid and preparation of GST fusion protein
The GalNAcT3 cDNA was cloned into pGEM-Teasy (Promega, Madison, WI, USA) by RT–PCR using the primers 5′-ATGGCTCACCTAAAGCGACTAG-3′ and 5′-GAAAGACTCCAGTCAAAATTTCC-3′, as described previously (Nomoto et al, 1999). The EcoRI-Eco47III fragment (amino acid residues 1–178 of the whole 324 amino acids) was cloned into EcoRI-SmaI sites in the pGEX-6P, and GST fusion protein (GST-GalNAcT3N) was purified according to the manufacturer's protocol(Pharmacia, Uppsala, Sweden).
Western blot analysis of GST fusion protein
Both GST and GST-GalNAcT3N were separated on a 12% SDS–PAGE gel and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA) using semidry blotter. Immunoblot analysis was performed with anti-GST antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-GalNAcT3 antibody, which was a rabbit polyclonal antibody against a synthesised peptide of human GalNAc-T3 (Nomoto et al, 1999) and was used for immunohistochemistry.
Immunohistochemistry for GalNAc-T3 expression
GalNAc-T3 expression was analysed by immunohistochemistry. The labelled streptavidin biotin method was used on 4-μm sections of formalin-fixed, paraffin-embedded tissues after deparaffinisation. Briefly, deparaffinised tissue sections were microwaved twice in 10 mM citrate buffer (pH 6.0) for 5 min to retrieve the antigenicity. The sections were incubated with normal rabbit serum to block the non-specific antibody binding sites. The sections were then incubated with a 1 : 5000 dilution of rabbit polyclonal antibody against a synthesised peptide of human GalNAc-T3 (Nomoto et al, 1999) or with control rabbit non-immunised serum at 4°C overnight. Immunostaining was performed by the biotin-streptavidin immunoperoxidase method with 3,3′-diaminobenzidine as a chromogen (SAB-PO kit; Nichirei, Tokyo, Japan). Sections were counterstained with methyl green. GalNAc-T3 expression in normal bronchial epithelial cells, bronchial gland cells, and alveolar pneumocytes in the same sections served as an internal positive control. The GalNAc-T3 expression in tumours was classified as high or low, according to the proportion of positively stained tumour cells. Tumours with staining in at least 50% of cancer cells, were judged as having high GalNAc-T3 expression (retaining expression of GalNAc-T3). Tumours with staining in less than 50% of cancer cells, were judged as having low GalNAc-T3 expression (losing expression of GalNAc-T3), as we previously described (Shibao et al, 2002).
For the Ki-67 staining, the results that were previously reported (Hommura et al, 2000) were used for the present study.
Statistical analysis
The associations between GalNAc-T3 expression and categorical variables were analysed by the χ2 test or Fisher's exact test as appropriate. The associations between GalNAc-T3 expression and age or the Ki-67 labelling index (LI) were analysed by Student's t-test. To simultaneously examine the effect of more than one factor on GalNAc-T3 expression, multivariate logistic regression analysis was used (Cox and Snell, 1989). The survival curves were estimated using the Kaplan-Meier method, and differences in survival distributions were evaluated by the generalised Wilcoxon test. Cox's proportional hazards modelling of factors potentially related to survival was performed to identify factors with a significant influence on survival. P values less than 0.05 were considered statistically significant. All tests were two-sided.
RESULTS
To confirm the availability of an anti-GalNAc-T3 polyclonal antibody, Western blot analysis of a GST fusion protein (GST-GalNAcT3N) was performed (Figure 1). Strong signals of the GST fusion protein were obtained in the immunoblotting with anti-GalNAc-T3 polyclonal antibody as well as in that with anti-GST antibody. The specificity of this anti-GalNAc-T3 polyclonal antibody was also tested by immunohistochemistry. After incubation of this antibody with the excess of GST-GalNAcT3N, the positive immunostaining was abolished (Figure 2).
Figure 1.
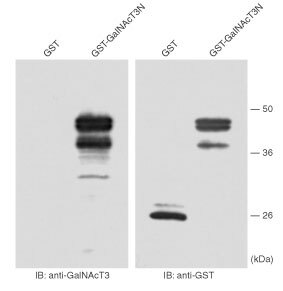
Western blotting of GST fusion protein. GST (100 ng) and GST-GalNAcT3N (200 ng) proteins were loaded on a 12% SDS–PAGE gel and transferred to membrane. Immunoblot analysis was performed with an anti-GalNAcT3 antibody (left) and anti-GST antibody (right). IB, immunoblotting.
Figure 2.
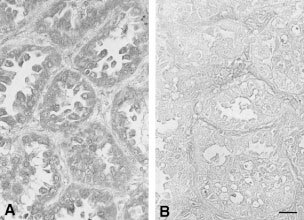
Immunoreactivity of an anti-GalNAc-T3 polyclonal antibody. Immunostaining was performed with this antibody in a NSCLC specimen after incubation of this antibody with excess of GST (A) or GST-GalNAcT3N (B). Scale bar=20 μm.
Typical immunostaining patterns for GalNAc-T3 with this antibody in NSCLCs are shown in Figure 3. In tumour cells, GalNAc-T3 expression was found diffusely in the cytoplasm, or localised in the Golgi apparatus. Normal bronchial epithelial cells (Figure 3A), bronchial gland cells (Figure 3B), and alveolar pneumocytes (data not shown) also expressed GalNAc-T3.
Figure 3.
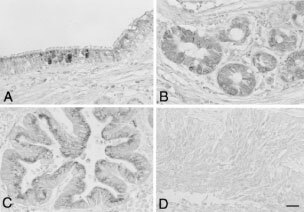
Immunohistochemical staining patterns for GalNAc-T3. Normal bronchial epithelial cells (A) and bronchial gland cells (B) showed GalNAc-T3 expression. GalNAc-T3 expression was found diffusely in the cytoplasm of tumour cells or localised in the Golgi apparatus in an adenocarcinoma (C), but not seen in a squamous cell carcinoma (D). Scale bar=20 μm.
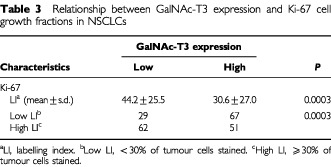
Of the 215 NSCLCs, 122 (56.7%) had high GalNAc-T3 expression, and 93 (43.3%) had low GalNAc-T3 expression (Table 1). The status of GalNAc-T3 expression was statistically analysed to investigate possible correlations with clinical and clinicopathological characteristics of NSCLCs. Low expression of GalNAc-T3 was significantly more prevalent in tumours from men than in those from women (P=0.0001 by the χ2 test) and in tumours from smokers compared to nonsmokers (P=0.001). Low GalNAc-T3 expression was also more prevalent in squamous cell carcinomas than nonsquamous cell carcinomas (P<0.0001) (Table 1). GalNAc-T3 expression was not associated with pTNM classifications and pStage. Multivariate logistic regression analysis showed a significant association of low GalNAc-T3 expression with squamous cell (P<0.0001) (Table 2). This relationship between GalNAc-T3 expression and histology was the only significant relationship between GalNAc-T3 expression and various factors within the context of the multivariate model. Tumours having low GalNAc-T3 expression showed a significantly higher Ki-67 LI than tumours having high GalNAc-T3 expression (P=0.0003) (Table 3). Tumours having low GalNAc-T3 expression also exhibited a high Ki-67 LI more frequently than tumours having high GalNAc-T3 expression (P=0.0003).
Table 1. Relationship between GalNAc-T3 expression and clinical and clinicopathological characteristics in 215 surgically resected NSCLCs.
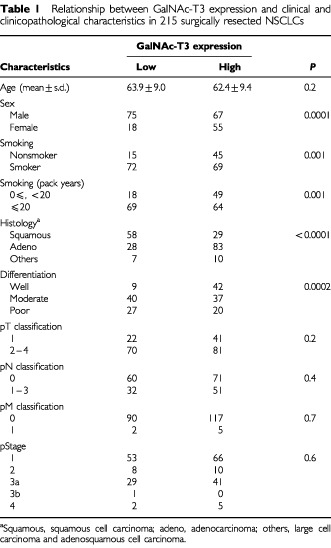
Table 2. Multivariate logistic regression analysis for the correlation between GalNAc-T3 expression and various characteristics.
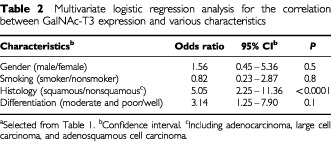
Table 3. Relationship between GalNAc-T3 expression and Ki-67 cell growth fractions in NSCLCs.
We analysed the relationship between GalNAc-T3 expression and postsurgical survival in pStage I NSCLCs (Figure 4). When the 103 pStage I NSCLCs were evaluated together, patients with tumours having low GalNAc-T3 expression survived a significantly shorter time than patients with tumours having high expression (5-year survival rates, 58% and 78%, respectively, by the Kaplan-Meier method; P=0.02 by the generalised Wilcoxon test) (Figure 4A). When only patients with 43 squamous cell carcinomas were analysed, GalNAc-T3 expression did not show a significant effect on survival (5-year survival rates, 54% for low GalNAc-T3 and 57% for high GalNAc-T3; P=0.8). Among 61 nonsquamous cell carcinoma patients, patients with tumours having low GalNAc-T3 expression survived a significantly shorter time than patients with tumours having high expression (5-year survival rates, 63% and 85%, respectively; P=0.03) (Figure 4B).
Figure 4.
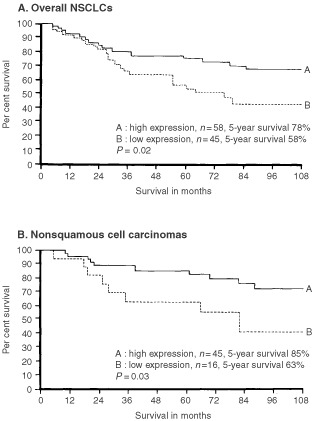
Kaplan–Meier survival curves of patients with pStage I NSCLCs stratified by GalNAc-T3 expression. Survival curves of patients with overall NSCLCs (A) and with nonsquamous cell carcinomas (B) are stratified by low and high GalNAc-T3 expression. Nonsquamous cell carcinoma included adenocarcinoma, large cell carcinoma and adenosquamous cell carcinoma.
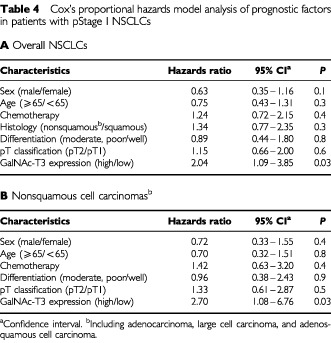
The importance of GalNAc-T3 as a prognostic factor was analysed by the Cox's proportional hazards model analysis in patients with pStage I NSCLCs (Table 4). Univariate analysis of potential prognostic factors revealed that low expression of GalNAc-T3 was the only statistically significant unfavourable prognostic factor in overall NSCLCs (hazards ratio, 2.04; P=0.03) and in nonsquamous cell carcinomas (hazards ratio, 2.70; P=0.03). GalNAC-T3 expression was not a prognostic factor in squamous cell carcinomas (hazards ratio, 0.91; P=0.8).
Table 4. Cox's proportional hazards model analysis of prognostic factors in patients with pStage I NSCLCsA Overall NSCLCs.
DISCUSSION
In the present study, GalNAc-T3 expression was frequently decreased in NSCLCs, although it was expressed in normal bronchial epithelial cells, bronchial gland cells and alveolar pneumocytes. Furthermore, low GalNAc-T3 expression of NSCLCs was associated with a shorter survival period, and was an unfavourable prognostic factor.
The finding that GalNAc-T3 expression was retained more frequently in nonsquamous cell carcinomas (most of which were adenocarcinomas) than in squamous cell carcinomas may reflect tissue specific expression of GalNAc-T3 in organs that contain secretory epithelial glands (Hagen et al, 1993; Homa et al, 1993; White et al, 1995; Bennett et al, 1996). Moreover, in nonsquamous cell carcinomas, decreased expression of GalNAc-T3 was associated with unfavourable prognosis. Consistent with these results, we have recently found that GalNAc-T3 is expressed in normal epithelial cells and gland cells of the colon, and that decreased expression of GalNAc-T3 is an unfavourable prognostic factor in adenocarcinomas of the colon (Shibao et al, 2002).
The mechanism underlying the relationship between decreased GalNAc-T3 expression and poor prognosis remains to be determined. Bronchial and alveolar epithelia, from which NSCLCs develop, normally express GalNAc-T3. Decreased GalNAc-T3 expression may induce decreased level of glycosylation in certain types of glycoprotein, resulting in altered functions of the glycoproteins. Therefore, the biological importance of GalNAc-T3 expression depends on the function of target substrate glycoproteins. The importance of GalNAc-T3 expression for the development and progression of cancer as well as for maintaining physiologic properties of normal cells also remains to be determined, although certain cancer-associated decreases or increases in glycosylation has been shown to directly contribute to cellular transformation (Vavasseur et al, 1994; Demetriou et al, 1995). Collectively, decreased expression of GalNAc-T3 may directly contribute to altered biological properties of NSCLCs. In fact, in this study, low expression of GalNAc-T3 in NSCLCs was associated with a higher LI of Ki-67 cell growth fractions, and resulted in shorter survival times.
In conclusion, these results indicate that GalNAc-T3 is a new marker of NSCLCs with specificity for histology and survival. GalNAc-T3 expression may be useful to stratify patients with pStage I tumours into groups at high and low risks of recurrence in NSCLCs, especially in nonsquamous cell carcinomas.
References
- American Joint Committee on Cancer1992LungInManual for staging of cancer4th ednBeahrs OH, Henson DE, Hutter RVP, Kennedy BJ (eds)pp15122Philadelphia: JB Lippincott Company [Google Scholar]
- BennettEPHassanHClausenH1996cDNA cloning and expression of a novel human UDP-N-acetyl-alpha-D-galactosamine. Polypeptide N-acetylgalactosaminyltransferase, GalNAc-t3 J Biol Chem 2711700617012 [DOI] [PubMed] [Google Scholar]
- BennettEPHassanHMandelUMirgorodskayaERoepstorffPBurchellJTaylor-PapadimitriouJHollingsworthMAMerkxGvan KesselAGEibergHSteffensenRClausenH1998Cloning of a human UDP-N-acetyl-alpha-D-Galactosamine:polypeptide N-acetylgalactosaminyltransferase that complements other GalNAc-transferases in complete O-glycosylation of the MUC1 tandem repeat J Biol Chem 2733047230481 [DOI] [PubMed] [Google Scholar]
- BennettEPHassanHMandelUHollingsworthMAAkisawaNIkematsuYMerkxGvan KesselAGOlofssonSClausenH1999Cloning and characterization of a close homologue of human UDP-N-acetyl-alpha-D-galactosamine:Polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy J Biol Chem 2742536225370 [DOI] [PubMed] [Google Scholar]
- CoxDSnellE1989Analysis of Binary Data2nd ednLondon: Chapman and Hall [Google Scholar]
- DemetriouMNabiIRCoppolinoMDedharSDennisJW1995Reduced contact-inhibition and substratum adhesion in epithelial cells expressing GlcNAc-transferase V J Cell Biol 130383392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosaka-AkitaHHommuraFMishinaTOguraSShimizuMKatohHKawakamiY2001A risk-stratification model of non-small cell lung cancers using cyclin E, Ki-67, and ras p21: different roles of G1 cyclins in cell proliferation and prognosis Cancer Res 6125002504 [PubMed] [Google Scholar]
- GibbsJB2000Mechanism-based target identification and drug discovery in cancer research Science 28719691973 [DOI] [PubMed] [Google Scholar]
- GinsbergRJVokesEERosenzweigK2001Non-small cell lung cancerInCancer: principles and practice of oncology6th ednDeVita VT, Hellma, S, Rosenberg SA (eds)pp925982Philadelphia: Lippincott-Raven Publishers [Google Scholar]
- HagenFKVan WuyckhuyseBTabakLA1993Purification, cloning, and expression of a bovine UDP-GalNAc:polypeptide N-acetyl-galactosaminyltransferase J Biol Chem 2681896018965 [PubMed] [Google Scholar]
- HakomoriS1989Aberrant glycosylation in tumours and tumour-associated carbohydrate antigens Adv Cancer Res 52257331 [DOI] [PubMed] [Google Scholar]
- HanahanDWeinbergRA2000The hallmarks of cancer Cell 1005770 [DOI] [PubMed] [Google Scholar]
- HarpoleJrDHHerndonIIJEWolfeWGIglehartJDMarksJR1995A prognostic model of recurrence and death in stage I non-small cell lung cancer utilizing presentation, histopatholoy, and oncoprotein expression Cancer Res 555156 [PubMed] [Google Scholar]
- HomaFLHollanderTLehmanDJThomsenDRElhammerAP1993Isolation and expression of a cDNA clone encoding a bovine UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase J Biol Chem 2681260912616 [PubMed] [Google Scholar]
- HommuraFDosaka-AkitaHMishinaTNishiMKojimaTHiroumiHOguraSShimizuMKatohHKawakamiY2000Prognostic significance of p27KIP1 protein and ki-67 growth fraction in non-small cell lung cancers Clin Cancer Res 640734081 [PubMed] [Google Scholar]
- KwiatkowskiDJHarpoleJrDHGoleskiJHerndonIIJEDar-BinSRichardsWBlancoRXuHStraussGMSugarbakerDJ1998Molecular pathologic substaging in 244 stage I non-small-cell lung cancer patients: clinical implications J Clin Oncol 1624682477 [DOI] [PubMed] [Google Scholar]
- NomotoMIzumiHIseTKatoKTakanoHNagataniGShibaoKOhtaRImamuraTKuwanoMMatsuoKYamadaYItohHKohnoK1999Structural basis for the regulation of UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyl transferase-3 gene expression in adenocarcinoma cells Cancer Res 5962146222 [PubMed] [Google Scholar]
- SekidoYFongKMMinnaJD2001Molecular biology of lung cancerInCancer: principles and practice of oncology6th ednDeVita VT, Hellman S, Rosenberg SA (eds)pp917924Philadelphia: Lippincott-Raven Publishers [Google Scholar]
- ShibaoKIzumiHNakayamaYOhtaRNagataNNomotoMMatsuoKYamadaYKitazawaKItohHKohnoK2002Expression of UDP-α-D-galactosamine: Polypeptide GalNAc N-acetylgalactosaminyltransferase-3 in relation to differentiation and prognosis in human colorectal carcinoma Cancer 9419391946 [DOI] [PubMed] [Google Scholar]
- StraussGMKwiatkowskiDJHarpoleDHLynchTJSkarinATSugarbakerDJ1995Molecular and pathologic markers in stage I non-small cell carcinoma of the lung J Clin Oncol 1312651279 [DOI] [PubMed] [Google Scholar]
- SutherlinMENishimoriICaffreyTBennettEPHassanHMandelUMackDIwamuraTClausenHHollingsworthMA1997Expression of three UDP-N-acetyl-alpha-D-galactosamine:polypeptide GalNAc N-acetylgalactosaminyltransferases in adenocarcinoma cell lines Cancer Res 5747444748 [PubMed] [Google Scholar]
- Ten HagenKGHagenFKBalysMMBeresTMVan WuyckhuyseBTabakLA1998Cloning and expression of a novel, tissue specifically expressed member of the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase family J Biol Chem 2732774927754 [DOI] [PubMed] [Google Scholar]
- VarkiA1993Biological roles of oligosaccharides: all of the theories are correct Glycobiology 397130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VavasseurFDoleKYangJMattaKLMyerscoughNCorfieldAParaskevaCBrockhausenI1994O-glycan biosynthesis in human colorectal adenoma cells during progression to cancer Eur J Biochem 222415424 [DOI] [PubMed] [Google Scholar]
- WandallHHHassanHMirgorodskayaEKristensenAKRoepstorffPBennetEPNielsenPAHollingsworthMABurchellJTaylor-PapadimitriouJClausenH1997Substrate specificities of three members of the human UDP-N-acetyl-alpha-D-galactosamine:Polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, -T2, and -T3 J Biol Chem 2722350323514 [DOI] [PubMed] [Google Scholar]
- WhiteTBennettEPTakioKSorensenTBondingNClausenH1995Purification and cDNA cloning of a human UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyl-transferase J Biol Chem 2702415624165 [DOI] [PubMed] [Google Scholar]
- World Health Organization1982Histological typing of lung tumours2nd ednAm J Clin Pathol 77123136 [DOI] [PubMed]


