Abstract
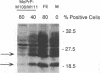
Mutations within a host cellular protein, PrP, have been associated with disease in the transmissible spongiform encephalopathies. Murine neuroblastoma cells persistently infected with mouse scrapie accumulate protease-resistant PrP (PrP-res), the abnormal form of PrP associated with disease in the transmissible spongiform encephalopathies. These cells provide a controlled system in which to study the molecular interactions which are important in the formation of PrP-res. We have expressed recombinant PrP molecules in mouse scrapie-infected murine neuroblastoma cells and assayed the effect of these heterologous PrP genes on the formation and accumulation of PrP-res. The results demonstrate that expression of heterologous PrP molecules which differ from the endogenous PrP by as little as one amino acid can profoundly interfere with the overall accumulation of PrP-res. The data suggest that precise interactions between homologous PrP molecules are important in PrP-res accumulation and that heterologous PrP molecules can block these interactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. M., Marsh R. F. The search for scrapie agent nucleic acid. Microbiol Rev. 1990 Sep;54(3):242–246. doi: 10.1128/mr.54.3.242-246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D. C., Bendheim P. E. A modified host protein model of scrapie. Ciba Found Symp. 1988;135:164–181. doi: 10.1002/9780470513613.ch11. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., Seligman S. J., Bablanian G., Windsor D., Scala L. J., Kim K. S., Chen C. M., Kascsak R. J., Bendheim P. E. Molecular location of a species-specific epitope on the hamster scrapie agent protein. J Virol. 1991 Jul;65(7):3667–3675. doi: 10.1128/jvi.65.7.3667-3675.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. E., McConnell I., Fraser H., Dickinson A. G. The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J Gen Virol. 1991 Mar;72(Pt 3):595–603. doi: 10.1099/0022-1317-72-3-595. [DOI] [PubMed] [Google Scholar]
- Büeler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993 Jul 2;73(7):1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Büeler H., Fischer M., Lang Y., Bluethmann H., Lipp H. P., DeArmond S. J., Prusiner S. B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992 Apr 16;356(6370):577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- Carlson G. A., Kingsbury D. T., Goodman P. A., Coleman S., Marshall S. T., DeArmond S., Westaway D., Prusiner S. B. Linkage of prion protein and scrapie incubation time genes. Cell. 1986 Aug 15;46(4):503–511. doi: 10.1016/0092-8674(86)90875-5. [DOI] [PubMed] [Google Scholar]
- Caughey B., Ernst D., Race R. E. Congo red inhibition of scrapie agent replication. J Virol. 1993 Oct;67(10):6270–6272. doi: 10.1128/jvi.67.10.6270-6272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Neary K., Buller R., Ernst D., Perry L. L., Chesebro B., Race R. E. Normal and scrapie-associated forms of prion protein differ in their sensitivities to phospholipase and proteases in intact neuroblastoma cells. J Virol. 1990 Mar;64(3):1093–1101. doi: 10.1128/jvi.64.3.1093-1101.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Raymond G. J., Ernst D., Race R. E. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J Virol. 1991 Dec;65(12):6597–6603. doi: 10.1128/jvi.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B. Scrapie associated PrP accumulation and its prevention: insights from cell culture. Br Med Bull. 1993 Oct;49(4):860–872. doi: 10.1093/oxfordjournals.bmb.a072651. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Britt W., Evans L., Wehrly K., Nishio J., Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983 May;127(1):134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Caughey B., Nishio J., Ernst D., Race R. Foreign PrP expression and scrapie infection in tissue culture cell lines. Dev Biol Stand. 1993;80:131–140. [PubMed] [Google Scholar]
- Come J. H., Fraser P. E., Lansbury P. T., Jr A kinetic model for amyloid formation in the prion diseases: importance of seeding. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5959–5963. doi: 10.1073/pnas.90.13.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer H., Gelderblom H., Hilmert H., Ozel M., Edelbluth C., Kimberlin R. H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- Doh-ura K., Tateishi J., Sasaki H., Kitamoto T., Sakaki Y. Pro----leu change at position 102 of prion protein is the most common but not the sole mutation related to Gerstmann-Sträussler syndrome. Biochem Biophys Res Commun. 1989 Sep 15;163(2):974–979. doi: 10.1016/0006-291x(89)92317-6. [DOI] [PubMed] [Google Scholar]
- Goldfarb L. G., Brown P., Goldgaber D., Garruto R. M., Yanagihara R., Asher D. M., Gajdusek D. C. Identical mutation in unrelated patients with Creutzfeldt-Jakob disease. Lancet. 1990 Jul 21;336(8708):174–175. doi: 10.1016/0140-6736(90)91693-5. [DOI] [PubMed] [Google Scholar]
- Hope J., Morton L. J., Farquhar C. F., Multhaup G., Beyreuther K., Kimberlin R. H. The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J. 1986 Oct;5(10):2591–2597. doi: 10.1002/j.1460-2075.1986.tb04539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K., Baker H. F., Crow T. J., Poulter M., Owen F., Terwilliger J. D., Westaway D., Ott J., Prusiner S. B. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature. 1989 Mar 23;338(6213):342–345. doi: 10.1038/338342a0. [DOI] [PubMed] [Google Scholar]
- Hsiao K., Meiner Z., Kahana E., Cass C., Kahana I., Avrahami D., Scarlato G., Abramsky O., Prusiner S. B., Gabizon R. Mutation of the prion protein in Libyan Jews with Creutzfeldt-Jakob disease. N Engl J Med. 1991 Apr 18;324(16):1091–1097. doi: 10.1056/NEJM199104183241604. [DOI] [PubMed] [Google Scholar]
- Hunter N., Hope J., McConnell I., Dickinson A. G. Linkage of the scrapie-associated fibril protein (PrP) gene and Sinc using congenic mice and restriction fragment length polymorphism analysis. J Gen Virol. 1987 Oct;68(Pt 10):2711–2716. doi: 10.1099/0022-1317-68-10-2711. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Tonna-DeMasi M., Fersko R., Carp R. I., Wisniewski H. M., Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987 Dec;61(12):3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin R. H., Cole S., Walker C. A. Temporary and permanent modifications to a single strain of mouse scrapie on transmission to rats and hamsters. J Gen Virol. 1987 Jul;68(Pt 7):1875–1881. doi: 10.1099/0022-1317-68-7-1875. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. A., Fraser H. The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J Gen Virol. 1989 Aug;70(Pt 8):2017–2025. doi: 10.1099/0022-1317-70-8-2017. [DOI] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol. 1977 Feb;34(2):295–304. doi: 10.1099/0022-1317-34-2-295. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Yamaguchi K., Doh-ura K., Tateishi J. A prion protein missense variant is integrated in kuru plaque cores in patients with Gerstmann-Sträussler syndrome. Neurology. 1991 Feb;41(2 ):306–310. doi: 10.1212/wnl.41.2_part_1.306. [DOI] [PubMed] [Google Scholar]
- Locht C., Chesebro B., Race R., Keith J. M. Molecular cloning and complete sequence of prion protein cDNA from mouse brain infected with the scrapie agent. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6372–6376. doi: 10.1073/pnas.83.17.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Scott M., Foster D., Pan K. M., Groth D., Mirenda C., Torchia M., Yang S. L., Serban D., Carlson G. A. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990 Nov 16;63(4):673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Race R. E., Caughey B., Graham K., Ernst D., Chesebro B. Analyses of frequency of infection, specific infectivity, and prion protein biosynthesis in scrapie-infected neuroblastoma cell clones. J Virol. 1988 Aug;62(8):2845–2849. doi: 10.1128/jvi.62.8.2845-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race R. E., Ernst D. Detection of proteinase K-resistant prion protein and infectivity in mouse spleen by 2 weeks after scrapie agent inoculation. J Gen Virol. 1992 Dec;73(Pt 12):3319–3323. doi: 10.1099/0022-1317-73-12-3319. [DOI] [PubMed] [Google Scholar]
- Race R. E., Fadness L. H., Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987 May;68(Pt 5):1391–1399. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- Race R. E., Graham K., Ernst D., Caughey B., Chesebro B. Analysis of linkage between scrapie incubation period and the prion protein gene in mice. J Gen Virol. 1990 Feb;71(Pt 2):493–497. doi: 10.1099/0022-1317-71-2-493. [DOI] [PubMed] [Google Scholar]
- Robertson M. N., Miyazawa M., Mori S., Caughey B., Evans L. H., Hayes S. F., Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and western blotting. J Virol Methods. 1991 Oct;34(3):255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- Robertson M. N., Spangrude G. J., Hasenkrug K., Perry L., Nishio J., Wehrly K., Chesebro B. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J Virol. 1992 Jun;66(6):3271–3277. doi: 10.1128/jvi.66.6.3271-3277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer R. G. The scrapie agent: "a virus by any other name". Curr Top Microbiol Immunol. 1991;172:195–232. [PubMed] [Google Scholar]
- Scott M. R., Köhler R., Foster D., Prusiner S. B. Chimeric prion protein expression in cultured cells and transgenic mice. Protein Sci. 1992 Aug;1(8):986–997. doi: 10.1002/pro.5560010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M., Foster D., Mirenda C., Serban D., Coufal F., Wälchli M., Torchia M., Groth D., Carlson G., DeArmond S. J. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989 Dec 1;59(5):847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- Scott M., Groth D., Foster D., Torchia M., Yang S. L., DeArmond S. J., Prusiner S. B. Propagation of prions with artificial properties in transgenic mice expressing chimeric PrP genes. Cell. 1993 Jun 4;73(5):979–988. doi: 10.1016/0092-8674(93)90275-u. [DOI] [PubMed] [Google Scholar]
- Westaway D., Goodman P. A., Mirenda C. A., McKinley M. P., Carlson G. A., Prusiner S. B. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987 Nov 20;51(4):651–662. doi: 10.1016/0092-8674(87)90134-6. [DOI] [PubMed] [Google Scholar]