Abstract
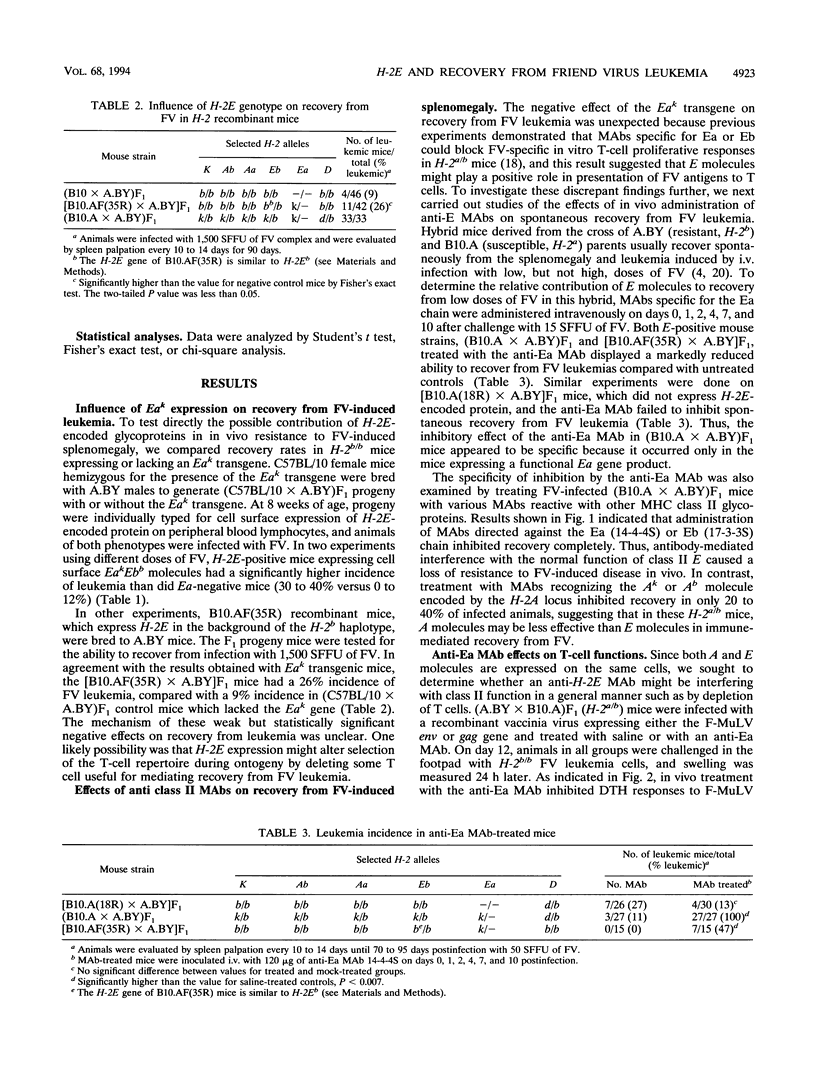
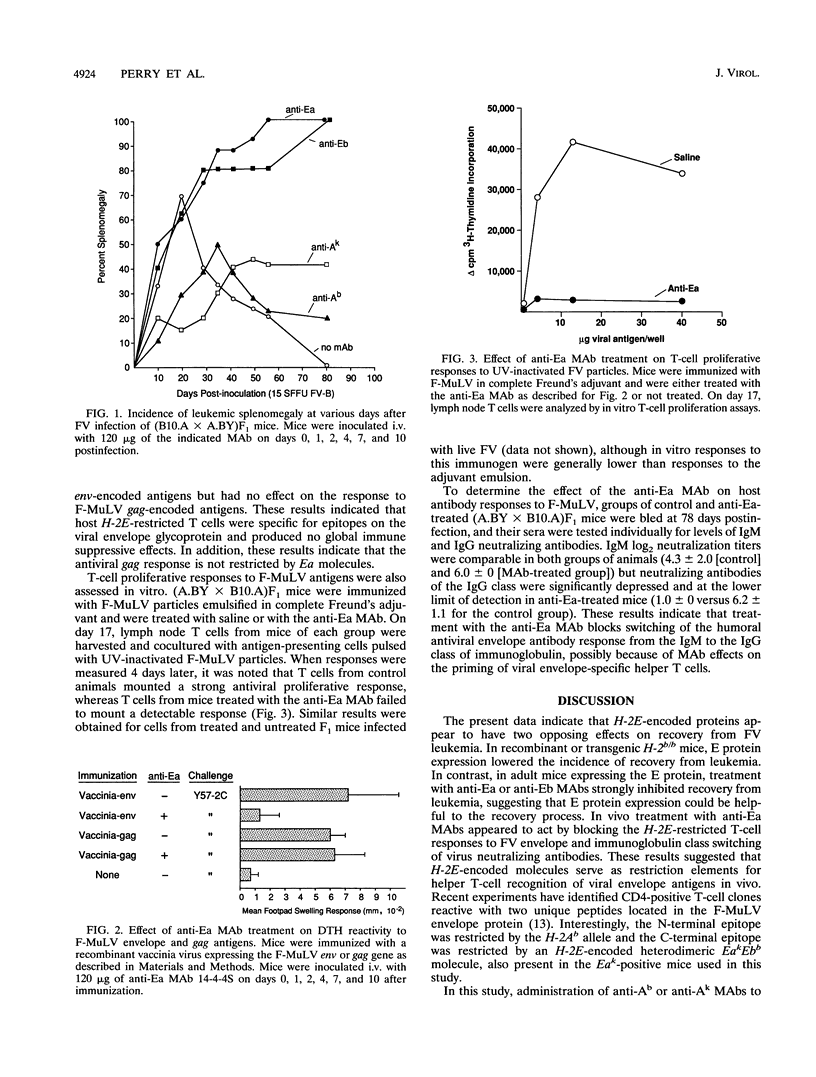
Resistance to erythroleukemia induced by infection with the Friend virus complex (FV) has been mapped to several genes residing both within and outside the murine major histocompatibility complex (MHC). MHC genes located in the A, D, and Qa/Tla regions of the murine H-2 complex have been shown to affect disease resistance through their capacity to regulate various aspects of the host immune response to viral antigens. This study establishes H-2E as the fourth MHC locus controlling immunological resistance to FV. Our investigation into the role of H-2E molecules revealed two distinct and opposite effects on recovery from Friend disease. H-2b/b mice normally lack a functional E gene product and are resistant to high doses of FV. The expression of H-2E molecules in H-2 recombinant or transgenic mice of this genotype resulted in a significant decrease in spontaneous recovery from FV-induced leukemia. In contrast, H-2E expression also appeared to influence recovery from Friend disease in a positive manner, since blocking these molecules with anti-E antibodies in vivo significantly decreased recovery from Friend disease. The data indicate that the positive effects of H-2E molecules derive from their function as restriction elements for helper T-cell recognition of the viral envelope glycoprotein, and we postulate that the negative effects are due to H-2E-dependent deletion in the T-cell repertoire during development.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Held W., Waanders G. A., Shakhov A. N., Scarpellino L., Lees R. K., MacDonald H. R. Exogenous and endogenous mouse mammary tumor virus superantigens. Immunol Rev. 1993 Feb;131:5–25. doi: 10.1111/j.1600-065x.1993.tb01527.x. [DOI] [PubMed] [Google Scholar]
- Anderson G. D., Banerjee S., David C. S. MHC class II A alpha and E alpha molecules determine the clonal deletion of V beta 6+ T cells. Studies with recombinant and transgenic mice. J Immunol. 1989 Dec 1;143(11):3757–3761. [PubMed] [Google Scholar]
- Chesebro B., Miyazawa M., Britt W. J. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Doig D., Nishio J. Antibody-induced modulation of Friend virus cell surface antigens decreases virus production by persistent erythroleukemia cells: influence of the Rfv-3 gene. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5784–5788. doi: 10.1073/pnas.76.11.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Identification of a non-H-2 gene (Rfv-3) influencing recovery from viremia and leukemia induced by Friend virus complex. Proc Natl Acad Sci U S A. 1979 Jan;76(1):425–429. doi: 10.1073/pnas.76.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Rfv-1 and Rfv-2, two H-2-associated genes that influence recovery from Friend leukemia virus-induced splenomegaly. J Immunol. 1978 Apr;120(4):1081–1085. [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Stimpfling J. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly: mapping of a gene within the major histocompatability complex. J Exp Med. 1974 Dec 1;140(6):1457–1467. doi: 10.1084/jem.140.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. K., Chesebro B. Spontaneous cessation of Friend murine leukemia virus production by leukemia cell line Y57: overgrowth by nonproducer cells. J Natl Cancer Inst. 1980 May;64(5):1153–1159. [PubMed] [Google Scholar]
- Doig D., Chesebro B. Anti-Friend virus antibody is associated with recovery from viremia and loss of viral leukemia cell-surface antigens in leukemic mice. Identification of Rfv-3 as a gene locus influencing antibody production. J Exp Med. 1979 Jul 1;150(1):10–19. doi: 10.1084/jem.150.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Morrison R. P., Wehrly K., Nishio J., Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986 Nov 7;234(4777):728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- Foo-Phillips M., Kozak C. A., Principato M. A., Abe R. Characterization of the Mlsf system. II. Identification of mouse mammary tumor virus proviruses involved in the clonal deletion of self-Mlsf-reactive T cells. J Immunol. 1992 Dec 1;149(11):3440–3447. [PubMed] [Google Scholar]
- Hasenkrug K. J., Stimpfling J. H. The antigenic properties of twenty-four new H-2 congenic recombinant mouse strains. Immunogenetics. 1986;24(6):423–427. doi: 10.1007/BF00377962. [DOI] [PubMed] [Google Scholar]
- Iwashiro M., Kondo T., Shimizu T., Yamagishi H., Takahashi K., Matsubayashi Y., Masuda T., Otaka A., Fujii N., Ishimoto A. Multiplicity of virus-encoded helper T-cell epitopes expressed on FBL-3 tumor cells. J Virol. 1993 Aug;67(8):4533–4542. doi: 10.1128/jvi.67.8.4533-4542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- Klein J., Benoist C., David C. S., Demant P., Lindahl K. F., Flaherty L., Flavell R. A., Hämmerling U., Hood L. E., Hunt S. W., 3rd Revised nomenclature of mouse H-2 genes. Immunogenetics. 1990;32(3):147–149. doi: 10.1007/BF02114967. [DOI] [PubMed] [Google Scholar]
- Mathis D. J., Benoist C., Williams V. E., 2nd, Kanter M., McDevitt H. O. Several mechanisms can account for defective E alpha gene expression in different mouse haplotypes. Proc Natl Acad Sci U S A. 1983 Jan;80(1):273–277. doi: 10.1073/pnas.80.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M., Nishio J., Chesebro B. Genetic control of T cell responsiveness to the Friend murine leukemia virus envelope antigen. Identification of class II loci of the H-2 as immune response genes. J Exp Med. 1988 Nov 1;168(5):1587–1605. doi: 10.1084/jem.168.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M., Nishio J., Chesebro B. Protection against Friend retrovirus-induced leukemia by recombinant vaccinia viruses expressing the gag gene. J Virol. 1992 Jul;66(7):4497–4507. doi: 10.1128/jvi.66.7.4497-4507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa M., Nishio J., Wehrly K., Chesebro B. Influence of MHC genes on spontaneous recovery from Friend retrovirus-induced leukemia. J Immunol. 1992 Jan 15;148(2):644–647. [PubMed] [Google Scholar]
- Miyazawa M., Nishio J., Wehrly K., David C. S., Chesebro B. Spontaneous recovery from Friend retrovirus-induced leukemia. Mapping of the Rfv-2 gene in the Q/TL region of mouse MHC. J Immunol. 1992 Mar 15;148(6):1964–1967. [PubMed] [Google Scholar]
- Miyazawa M., Nishio J., Wehrly K., Jay G., Melvold R. W., Chesebro B. Detailed mapping of the Rfv-1 gene that influences spontaneous recovery from Friend retrovirus-induced leukaemia. Eur J Immunogenet. 1992 Jun;19(3):159–164. doi: 10.1111/j.1744-313x.1992.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Miyazawa M., Nose M., Kawashima M., Kyogoku M. Pathogenesis of arteritis of SL/Ni mice. Possible lytic effect of anti-gp70 antibodies on vascular smooth muscle cells. J Exp Med. 1987 Oct 1;166(4):890–908. doi: 10.1084/jem.166.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Earl P. L., Nishio J., Lodmell D. L., Moss B., Chesebro B. Different H-2 subregions influence immunization against retrovirus and immunosuppression. Nature. 1987 Oct 22;329(6141):729–732. doi: 10.1038/329729a0. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Nishio J., Chesebro B. Influence of the murine MHC (H-2) on Friend leukemia virus-induced immunosuppression. J Exp Med. 1986 Feb 1;163(2):301–314. doi: 10.1084/jem.163.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Perry L. L., Barzaga M. E. Kinetics and specificity of T and B cell responses in relapsing experimental allergic encephalomyelitis. J Immunol. 1987 Mar 1;138(5):1434–1441. [PubMed] [Google Scholar]
- Perry L. L., Hotchkiss J. D., Lodmell D. L. Murine susceptibility to street rabies virus is unrelated to induction of host lymphoid depletion. J Immunol. 1990 May 1;144(9):3552–3557. [PubMed] [Google Scholar]
- Perry L. L., Williams I. R. Regulation of transplantation immunity in vivo by monoclonal antibodies recognizing host class II restriction elements. I. Genetics and specificity of anti-Ia immunotherapy in murine skin allograft recipients. J Immunol. 1985 May;134(5):2935–2941. [PubMed] [Google Scholar]
- Robertson M. N., Miyazawa M., Mori S., Caughey B., Evans L. H., Hayes S. F., Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and western blotting. J Virol Methods. 1991 Oct;34(3):255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- Robertson M. N., Spangrude G. J., Hasenkrug K., Perry L., Nishio J., Wehrly K., Chesebro B. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J Virol. 1992 Jun;66(6):3271–3277. doi: 10.1128/jvi.66.6.3271-3277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon M., Sola B., Evans L., Nishio J., Hayes S. F., Nathanson K., Garon C. F., Chesebro B. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell. 1986 Dec 26;47(6):851–859. doi: 10.1016/0092-8674(86)90800-7. [DOI] [PubMed] [Google Scholar]
- Tomonari K., Fairchild S., Rosenwasser O. A. Influence of viral superantigens on V beta- and V alpha-specific positive and negative selection. Immunol Rev. 1993 Feb;131:131–168. doi: 10.1111/j.1600-065x.1993.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Turner S. K., Miller C. L., Wettstein P. J., Hasenkrug K. J., Stimpfling J. H., Carlson G. A. Meiotic recombination within the H-2K-H-2D interval: characterization of a panel of congenic mice, including 12 new strains, using DNA markers. Immunogenetics. 1993;38(5):332–340. doi: 10.1007/BF00210474. [DOI] [PubMed] [Google Scholar]



