Abstract
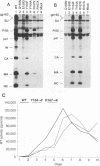
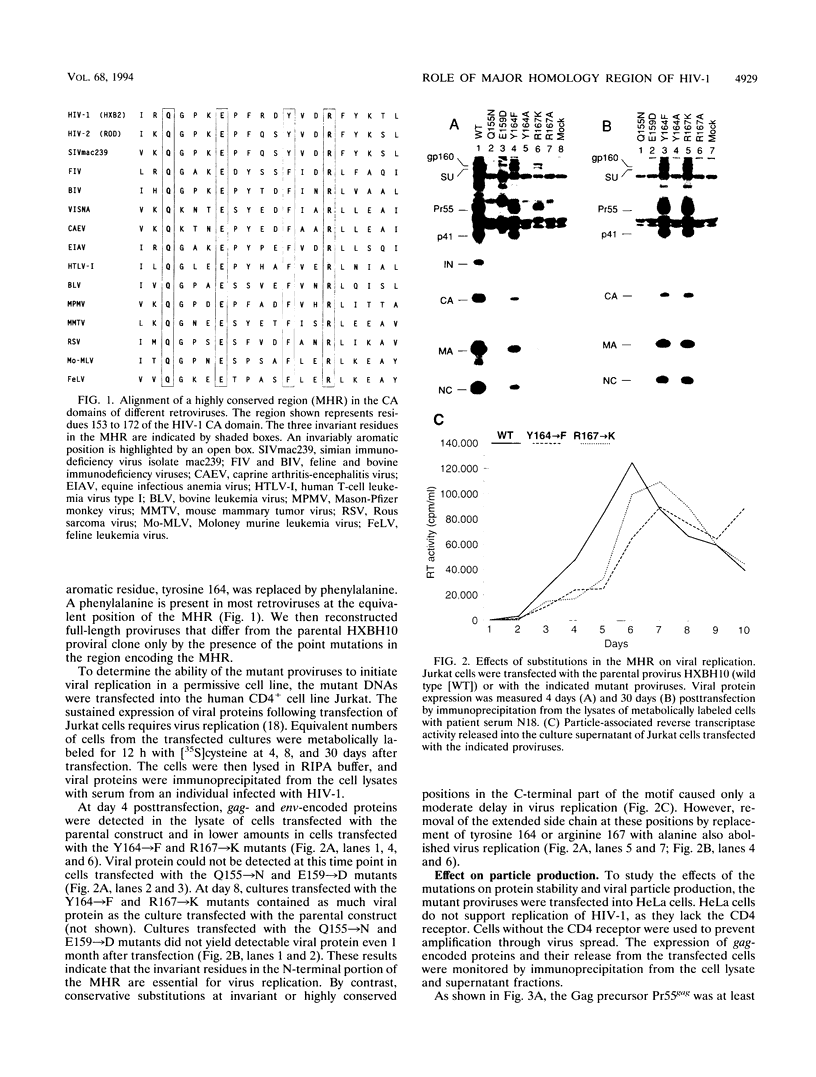
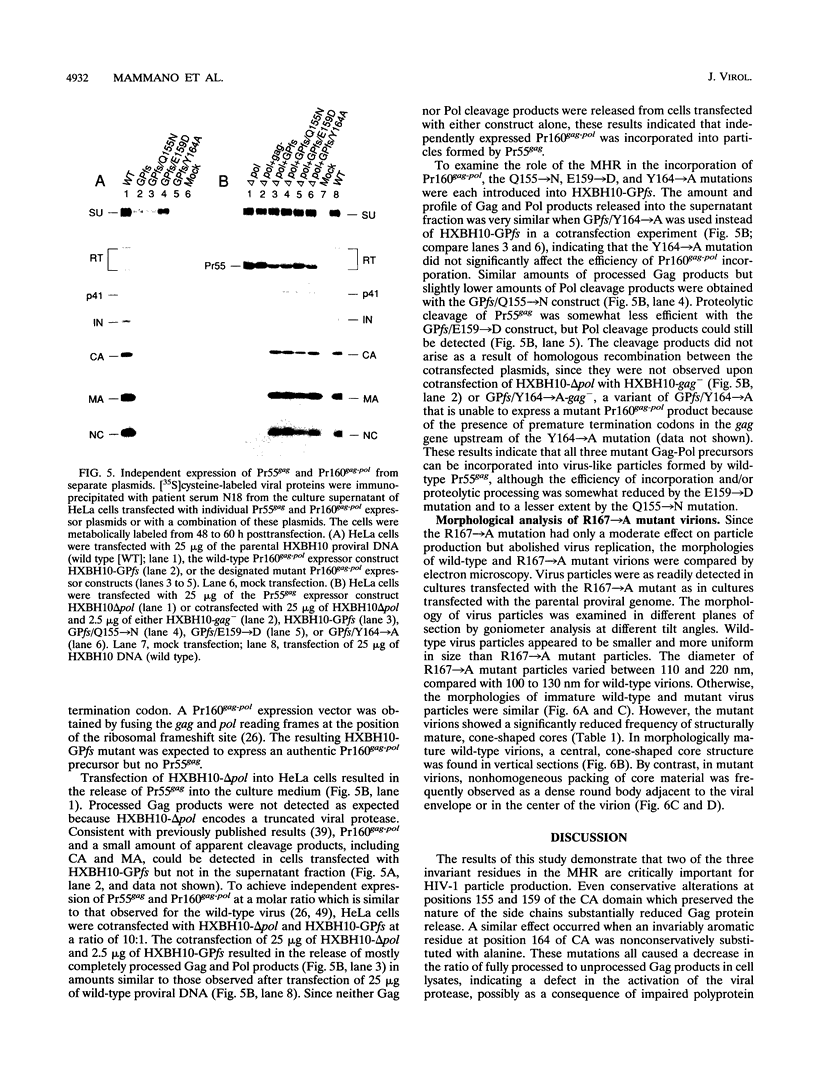
Retroviral capsid (CA) proteins contain a uniquely conserved stretch of 20 amino acids which has been named the major homology region (MHR). To examine the role of this region in human immunodeficiency virus type 1 morphogenesis and replication, four highly conserved positions in the MHR were individually altered by site-directed mutagenesis. Conservative substitution of two invariant residues (glutamine 155 and glutamic acid 159) abolished viral replication and significantly reduced the particle-forming ability of the mutant gag gene products. Conservative substitution of the third invariant residue in the MHR (arginine 167) or of an invariably aromatic residue (tyrosine 164) had only a moderate effect. However, removal of the extended side chains of these amino acids by substitution with alanine prevented viral replication and affected virion morphogenesis. The replacement of tyrosine 164 with alanine substantially impaired viral particle production. By contrast, the substitution of arginine 167 with alanine had only a two- to threefold effect on particle yield but led to the formation of aberrant core structures. The MHR mutant which were severely defective for particle production had a dominant negative effect on particle formation by the wild-type Gag product. The role of the MHR in the incorporation of the Gag-Pol precursor was examined by expressing the Gag and Gag-Pol polyproteins individually from separate plasmids. Only when the two precursor polyproteins were coexpressed did processed Gag and Pol products appear in the external medium. The appearance of these products was unaffected or only moderately affected by substitutions in the MHR of the Gag-Pol precursor, suggesting that the mutant Gag-Pol precursors were efficiently incorporated into viral particles. The results of this study indicate that specific residues within the MHR are required both for human immunodeficiency virus type 1 particle assembly and for the correct assembly of the viral core. However, mutant Gag and Gag-Pol polyproteins with substitutions in the MHR retained the ability to interact with wild-type Gag protein.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., Young R. A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990 May;64(5):1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz R. D., Luban J., Goff S. P. Specific binding of human immunodeficiency virus type 1 gag polyprotein and nucleocapsid protein to viral RNAs detected by RNA mobility shift assays. J Virol. 1993 Dec;67(12):7190–7200. doi: 10.1128/jvi.67.12.7190-7200.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M., Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990 Jan;87(2):523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M. I., Haggerty S., Dempsey M. P., Sharova N., Adzhubel A., Spitz L., Lewis P., Goldfarb D., Emerman M., Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993 Oct 14;365(6447):666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazal N., Carrière C., Gay B., Boulanger P. Phenotypic characterization of insertion mutants of the human immunodeficiency virus type 1 Gag precursor expressed in recombinant baculovirus-infected cells. J Virol. 1994 Jan;68(1):111–122. doi: 10.1128/jvi.68.1.111-122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Dayton A. I., Sodroski J. G., Rosen C. A., Goh W. C., Haseltine W. A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986 Mar 28;44(6):941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- Dorfman T., Luban J., Goff S. P., Haseltine W. A., Göttlinger H. G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993 Oct;67(10):6159–6169. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman T., Mammano F., Haseltine W. A., Göttlinger H. G. Role of the matrix protein in the virion association of the human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1994 Mar;68(3):1689–1696. doi: 10.1128/jvi.68.3.1689-1696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich L. S., Agresta B. E., Carter C. A. Assembly of recombinant human immunodeficiency virus type 1 capsid protein in vitro. J Virol. 1992 Aug;66(8):4874–4883. doi: 10.1128/jvi.66.8.4874-4883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fäcke M., Janetzko A., Shoeman R. L., Kräusslich H. G. A large deletion in the matrix domain of the human immunodeficiency virus gag gene redirects virus particle assembly from the plasma membrane to the endoplasmic reticulum. J Virol. 1993 Aug;67(8):4972–4980. doi: 10.1128/jvi.67.8.4972-4980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H. R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991 Jun;5(6):617–637. [PubMed] [Google Scholar]
- Gelderblom H. R., Hausmann E. H., Ozel M., Pauli G., Koch M. A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987 Jan;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989 Oct 6;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- González S. A., Affranchino J. L., Gelderblom H. R., Burny A. Assembly of the matrix protein of simian immunodeficiency virus into virus-like particles. Virology. 1993 Jun;194(2):548–556. doi: 10.1006/viro.1993.1293. [DOI] [PubMed] [Google Scholar]
- Gorelick R. J., Nigida S. M., Jr, Bess J. W., Jr, Arthur L. O., Henderson L. E., Rein A. Noninfectious human immunodeficiency virus type 1 mutants deficient in genomic RNA. J Virol. 1990 Jul;64(7):3207–3211. doi: 10.1128/jvi.64.7.3207-3211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Dorfman T., Cohen E. A., Haseltine W. A. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7381–7385. doi: 10.1073/pnas.90.15.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Dorfman T., Sodroski J. G., Haseltine W. A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar O., Garrigues J., Travis B., Moran P., Zarling J., Hu S. L. Human immunodeficiency virus-like, nonreplicating, gag-env particles assemble in a recombinant vaccinia virus expression system. J Virol. 1990 Jun;64(6):2653–2659. doi: 10.1128/jvi.64.6.2653-2659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Arya S. K., Popovic M., Gallo R. C., Wong-Staal F. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature. 1984 Nov 8;312(5990):166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- Hansen M., Jelinek L., Whiting S., Barklis E. Transport and assembly of gag proteins into Moloney murine leukemia virus. J Virol. 1990 Nov;64(11):5306–5316. doi: 10.1128/jvi.64.11.5306-5316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Bowers M. A., Sowder R. C., 2nd, Serabyn S. A., Johnson D. G., Bess J. W., Jr, Arthur L. O., Bryant D. K., Fenselau C. Gag proteins of the highly replicative MN strain of human immunodeficiency virus type 1: posttranslational modifications, proteolytic processings, and complete amino acid sequences. J Virol. 1992 Apr;66(4):1856–1865. doi: 10.1128/jvi.66.4.1856-1865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. W., Schwartzberg P., Goff S. P. Point mutations in the P30 domain of the gag gene of Moloney murine leukemia virus. Virology. 1985 Apr 15;142(1):211–214. doi: 10.1016/0042-6822(85)90435-0. [DOI] [PubMed] [Google Scholar]
- Höglund S., Ofverstedt L. G., Nilsson A., Lundquist P., Gelderblom H., Ozel M., Skoglund U. Spatial visualization of the maturing HIV-1 core and its linkage to the envelope. AIDS Res Hum Retroviruses. 1992 Jan;8(1):1–7. doi: 10.1089/aid.1992.8.1. [DOI] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Klimkait T., Strebel K., Hoggan M. D., Martin M. A., Orenstein J. M. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J Virol. 1990 Feb;64(2):621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lu Y. L., Spearman P., Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993 Nov;67(11):6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luban J., Goff S. P. Binding of human immunodeficiency virus type 1 (HIV-1) RNA to recombinant HIV-1 gag polyprotein. J Virol. 1991 Jun;65(6):3203–3212. doi: 10.1128/jvi.65.6.3203-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis R. J., Ahmad N., Lillehoj E. P., Raum M. G., Salazar F. H., Chan H. W., Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988 Nov;62(11):3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Morrow C. D. Overexpression of the gag-pol precursor from human immunodeficiency virus type 1 proviral genomes results in efficient proteolytic processing in the absence of virion production. J Virol. 1991 Sep;65(9):5111–5117. doi: 10.1128/jvi.65.9.5111-5117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Morrow C. D. The nonmyristylated Pr160gag-pol polyprotein of human immunodeficiency virus type 1 interacts with Pr55gag and is incorporated into viruslike particles. J Virol. 1992 Nov;66(11):6304–6313. doi: 10.1128/jvi.66.11.6304-6313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton W., Connor R. I., Landau N. R. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of gag and mutational analysis. J Virol. 1993 Dec;67(12):7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Ross E. K., Fuerst T. R., Orenstein J. M., O'Neill T., Martin M. A., Venkatesan S. Maturation of human immunodeficiency virus particles assembled from the gag precursor protein requires in situ processing by gag-pol protease. AIDS Res Hum Retroviruses. 1991 May;7(5):475–483. doi: 10.1089/aid.1991.7.475. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Gordon M. L., Goff S. P. Mutations in the gag gene of Moloney murine leukemia virus: effects on production of virions and reverse transcriptase. J Virol. 1984 Mar;49(3):918–924. doi: 10.1128/jvi.49.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., Srinivasakumar N., Hammarskjöld M. L., Rekosh D. Requirements for incorporation of Pr160gag-pol from human immunodeficiency virus type 1 into virus-like particles. J Virol. 1993 Apr;67(4):2266–2275. doi: 10.1128/jvi.67.4.2266-2275.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W. Assembly of animal viruses at cellular membranes. Annu Rev Microbiol. 1988;42:489–516. doi: 10.1146/annurev.mi.42.100188.002421. [DOI] [PubMed] [Google Scholar]
- Strambio-de-Castillia C., Hunter E. Mutational analysis of the major homology region of Mason-Pfizer monkey virus by use of saturation mutagenesis. J Virol. 1992 Dec;66(12):7021–7032. doi: 10.1128/jvi.66.12.7021-7032.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger E. F., Cohen E. A., Lu Y. C., Sodroski J. G., Haseltine W. A. Functional role of human immunodeficiency virus type 1 vpu. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. T., Barklis E. Assembly, processing, and infectivity of human immunodeficiency virus type 1 gag mutants. J Virol. 1993 Jul;67(7):4264–4273. doi: 10.1128/jvi.67.7.4264-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon R. A., Jr, Wills J. W. Characterization of a small (25-kilodalton) derivative of the Rous sarcoma virus Gag protein competent for particle release. J Virol. 1993 Sep;67(9):5550–5561. doi: 10.1128/jvi.67.9.5550-5561.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C. Form, function, and use of retroviral gag proteins. AIDS. 1991 Jun;5(6):639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Weldon R. A., Jr, Nelle T. D., Erdie C. R. Suppression of retroviral MA deletions by the amino-terminal membrane-binding domain of p60src. J Virol. 1991 Jul;65(7):3804–3812. doi: 10.1128/jvi.65.7.3804-3812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W., Braddock M., Adams S. E., Rathjen P. D., Kingsman S. M., Kingsman A. J. HIV expression strategies: ribosomal frameshifting is directed by a short sequence in both mammalian and yeast systems. Cell. 1988 Dec 23;55(6):1159–1169. doi: 10.1016/0092-8674(88)90260-7. [DOI] [PubMed] [Google Scholar]
- Yao X. J., Göttlinger H., Haseltine W. A., Cohen E. A. Envelope glycoprotein and CD4 independence of vpu-facilitated human immunodeficiency virus type 1 capsid export. J Virol. 1992 Aug;66(8):5119–5126. doi: 10.1128/jvi.66.8.5119-5126.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Yuan X., Matsuda Z., Lee T. H., Essex M. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J Virol. 1992 Aug;66(8):4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Yu X., Lee T. H., Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993 Nov;67(11):6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Poblotzki A., Wagner R., Niedrig M., Wanner G., Wolf H., Modrow S. Identification of a region in the Pr55gag-polyprotein essential for HIV-1 particle formation. Virology. 1993 Apr;193(2):981–985. doi: 10.1006/viro.1993.1210. [DOI] [PubMed] [Google Scholar]