Abstract
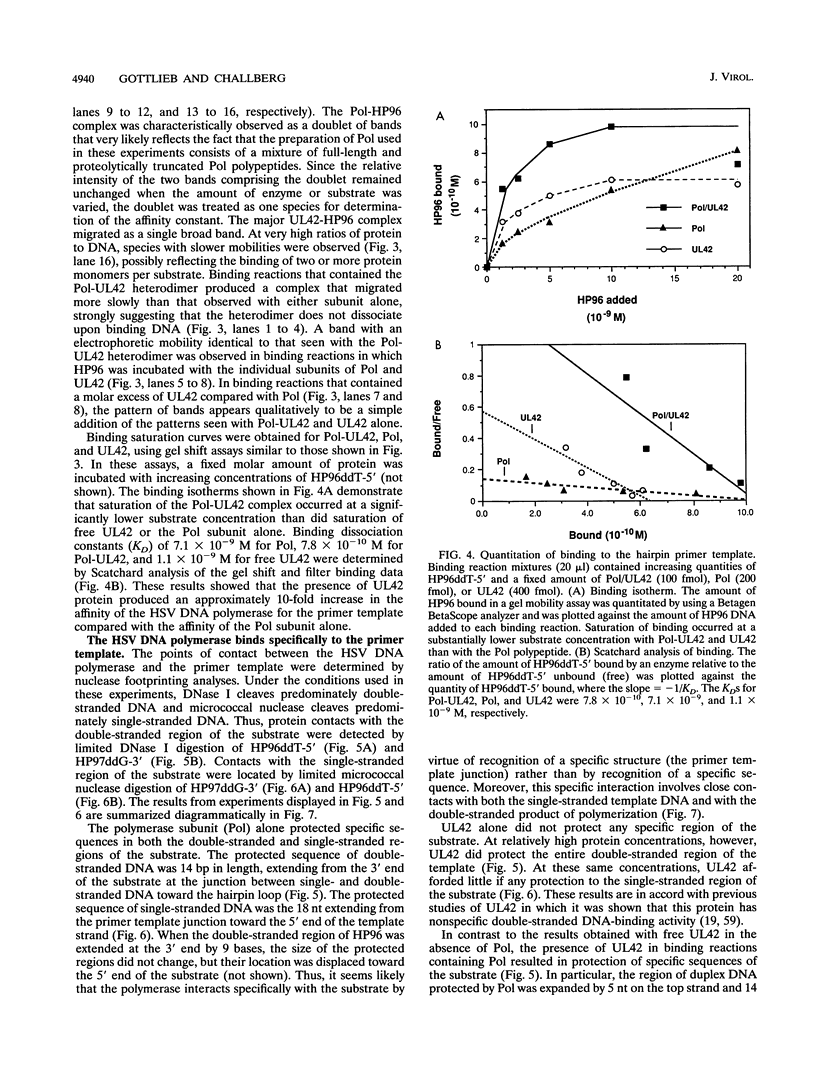
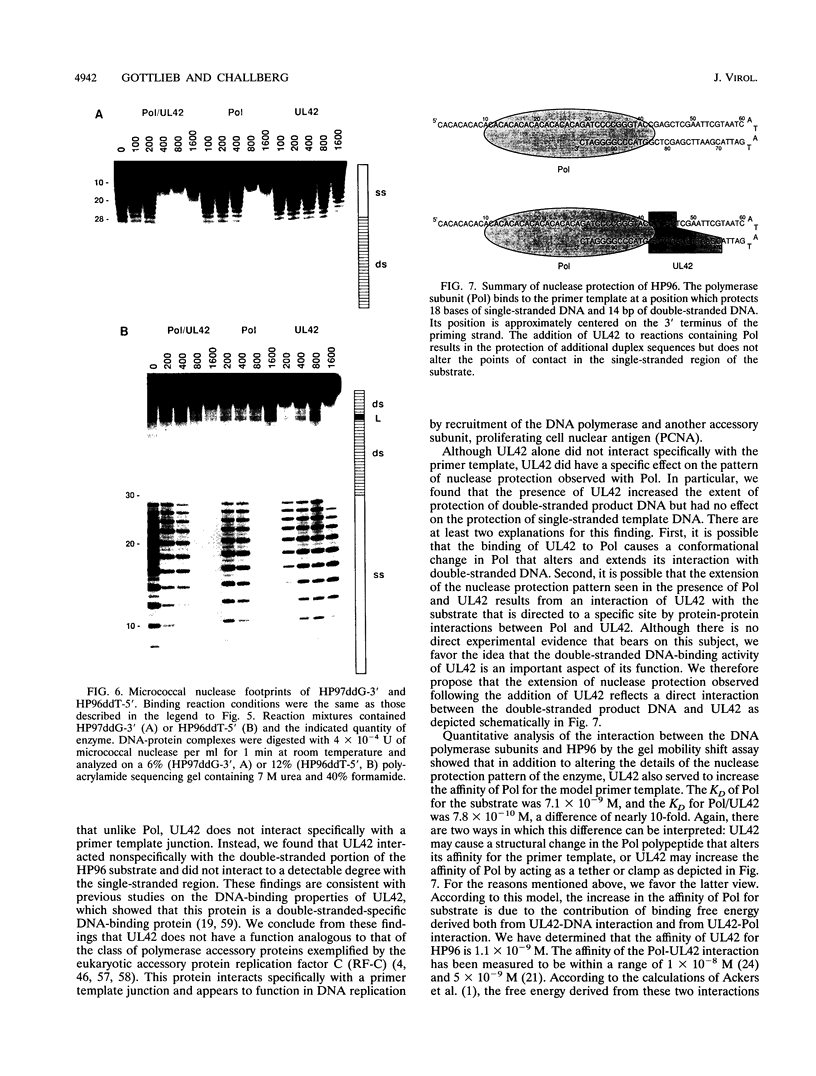
Genetic and biochemical studies have shown that the products of the herpes simplex virus type 1 (HSV-1) DNA polymerase (UL30) and UL42 genes are both required for viral DNA replication. A number of studies have previously suggested that these two proteins specifically interact, and more recent studies have confirmed that the viral DNA polymerase from HSV-1-infected cells consists of a heterodimer of the UL30 (Pol; the catalytic subunit) and UL42 polypeptides. A comparison of the catalytic properties of the Pol-UL42 complex with those of the isolated subunits of the enzyme purified from recombinant baculovirus-infected insect cells indicated that the Pol-UL42 complex is more highly processive than Pol alone on singly primed M13 single-stranded substrates. The results of these studies are consistent with the idea that the UL42 polypeptide is an accessory subunit of the HSV-1 DNA polymerase that acts to increase the processivity of polymerization. Preliminary experiments suggested that the increase in processivity was accompanied by an increase in the affinity of the polymerase for the ends of linear duplex DNA. We have further characterized the effect of the UL42 polypeptide on a defined hairpin primer template substrate. Gel shift and filter binding studies show that the affinity of the Pol catalytic subunit for the 3' terminus of the primer template increases 10-fold in the presence of UL42. DNase I footprinting experiments indicate that the Pol catalytic subunit binds to the primer template at a position that protects 14 bp of the 3' duplex region and an adjacent 18 bases of the single-stranded template. The presence of the UL42 polypeptide results in the additional protection of a contiguous 5 to 14 bp in the duplex region but does not affect the 5' position of the Pol subunit. Free UL42 protects the entire duplex region of the substrate but does not bind to the single-stranded region. Taken together, these results suggest that the increase in processivity in the presence of UL42 is related to the double-stranded DNA-binding activity of free UL42 and that the role of UL42 in the DNA polymerase complex is to act as a clamp, decreasing the probability that the polymerase will dissociate from the template after each cycle of catalysis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Shea M. A., Smith F. R. Free energy coupling within macromolecules. The chemical work of ligand binding at the individual sites in co-operative systems. J Mol Biol. 1983 Oct 15;170(1):223–242. doi: 10.1016/s0022-2836(83)80234-4. [DOI] [PubMed] [Google Scholar]
- Beese L. S., Derbyshire V., Steitz T. A. Structure of DNA polymerase I Klenow fragment bound to duplex DNA. Science. 1993 Apr 16;260(5106):352–355. doi: 10.1126/science.8469987. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Burgers P. M. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases delta and epsilon. J Biol Chem. 1991 Nov 25;266(33):22698–22706. [PubMed] [Google Scholar]
- Carmichael E. P., Kosovsky M. J., Weller S. K. Isolation and characterization of herpes simplex virus type 1 host range mutants defective in viral DNA synthesis. J Virol. 1988 Jan;62(1):91–99. doi: 10.1128/jvi.62.1.91-99.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael E. P., Weller S. K. Herpes simplex virus type 1 DNA synthesis requires the product of the UL8 gene: isolation and characterization of an ICP6::lacZ insertion mutation. J Virol. 1989 Feb;63(2):591–599. doi: 10.1128/jvi.63.2.591-599.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartrand P., Crumpacker C. S., Schaffer P. A., Wilkie N. M. Physical and genetic analysis of the herpes simplex virus DNA polymerase locus. Virology. 1980 Jun;103(2):311–326. doi: 10.1016/0042-6822(80)90190-7. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Aschman D. P., Gelep P. T., Retondo M. J., Weller S. K., Schaffer P. A. Fine mapping and molecular cloning of mutations in the herpes simplex virus DNA polymerase locus. J Virol. 1984 Jan;49(1):236–247. doi: 10.1128/jvi.49.1.236-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley A. J., Knipe D. M., Jones P. C., Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981 Jan;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crute J. J., Lehman I. R. Herpes simplex-1 DNA polymerase. Identification of an intrinsic 5'----3' exonuclease with ribonuclease H activity. J Biol Chem. 1989 Nov 15;264(32):19266–19270. [PubMed] [Google Scholar]
- Digard P., Bebrin W. R., Weisshart K., Coen D. M. The extreme C terminus of herpes simplex virus DNA polymerase is crucial for functional interaction with processivity factor UL42 and for viral replication. J Virol. 1993 Jan;67(1):398–406. doi: 10.1128/jvi.67.1.398-406.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digard P., Chow C. S., Pirrit L., Coen D. M. Functional analysis of the herpes simplex virus UL42 protein. J Virol. 1993 Mar;67(3):1159–1168. doi: 10.1128/jvi.67.3.1159-1168.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digard P., Coen D. M. A novel functional domain of an alpha-like DNA polymerase. The binding site on the herpes simplex virus polymerase for the viral UL42 protein. J Biol Chem. 1990 Oct 15;265(29):17393–17396. [PubMed] [Google Scholar]
- Dorsky D. I., Crumpacker C. S. Expression of herpes simplex virus type 1 DNA polymerase gene by in vitro translation and effects of gene deletions on activity. J Virol. 1988 Sep;62(9):3224–3232. doi: 10.1128/jvi.62.9.3224-3232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B. Acyclovir: discovery, mechanism of action, and selectivity. J Med Virol. 1993;Suppl 1:2–6. doi: 10.1002/jmv.1890410503. [DOI] [PubMed] [Google Scholar]
- Freemont P. S., Friedman J. M., Beese L. S., Sanderson M. R., Steitz T. A. Cocrystal structure of an editing complex of Klenow fragment with DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8924–8928. doi: 10.1073/pnas.85.23.8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo M. L., Jackwood D. H., Murphy M., Marsden H. S., Parris D. S. Purification of the herpes simplex virus type 1 65-kilodalton DNA-binding protein: properties of the protein and evidence of its association with the virus-encoded DNA polymerase. J Virol. 1988 Aug;62(8):2874–2883. doi: 10.1128/jvi.62.8.2874-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., DiTusa S. F., Cordingley M. G. The C-terminal third of UL42, a HSV-1 DNA replication protein, is dispensable for viral growth. Virology. 1993 Jun;194(2):647–653. doi: 10.1006/viro.1993.1304. [DOI] [PubMed] [Google Scholar]
- Gottlieb J., Marcy A. I., Coen D. M., Challberg M. D. The herpes simplex virus type 1 UL42 gene product: a subunit of DNA polymerase that functions to increase processivity. J Virol. 1990 Dec;64(12):5976–5987. doi: 10.1128/jvi.64.12.5976-5987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffey M. L., Stevens J. T., Terry B. J., Dorsky D. I., Crumpacker C. S., Wietstock S. M., Ruyechan W. T., Field A. K. Expression of herpes simplex virus type 1 DNA polymerase in Saccharomyces cerevisiae and detection of virus-specific enzyme activity in cell-free lysates. J Virol. 1988 Dec;62(12):4493–4498. doi: 10.1128/jvi.62.12.4493-4498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatake R. K., Bifano M., Tenney D. J., Hurlburt W. W., Cordingley M. G. The herpes simplex virus type 1 DNA polymerase accessory protein, UL42, contains a functional protease-resistant domain. J Gen Virol. 1993 Oct;74(Pt 10):2181–2189. doi: 10.1099/0022-1317-74-10-2181. [DOI] [PubMed] [Google Scholar]
- Hernandez T. R., Lehman I. R. Functional interaction between the herpes simplex-1 DNA polymerase and UL42 protein. J Biol Chem. 1990 Jul 5;265(19):11227–11232. [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E., Alberts B. M. Two types of replication proteins increase the rate at which T4 DNA polymerase traverses the helical regions in a single-stranded DNA template. J Biol Chem. 1981 Apr 25;256(8):4087–4094. [PubMed] [Google Scholar]
- Huber H. E., Tabor S., Richardson C. C. Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J Biol Chem. 1987 Nov 25;262(33):16224–16232. [PubMed] [Google Scholar]
- Ito J., Braithwaite D. K. Compilation and alignment of DNA polymerase sequences. Nucleic Acids Res. 1991 Aug 11;19(15):4045–4057. doi: 10.1093/nar/19.15.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis T. C., Newport J. W., von Hippel P. H. Stimulation of the processivity of the DNA polymerase of bacteriophage T4 by the polymerase accessory proteins. The role of ATP hydrolysis. J Biol Chem. 1991 Jan 25;266(3):1830–1840. [PubMed] [Google Scholar]
- Johnson P. A., Best M. G., Friedmann T., Parris D. S. Isolation of a herpes simplex virus type 1 mutant deleted for the essential UL42 gene and characterization of its null phenotype. J Virol. 1991 Feb;65(2):700–710. doi: 10.1128/jvi.65.2.700-710.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf K. W. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur J Biochem. 1979 Jul;98(1):231–244. doi: 10.1111/j.1432-1033.1979.tb13181.x. [DOI] [PubMed] [Google Scholar]
- Kuriyan J., O'Donnell M. Sliding clamps of DNA polymerases. J Mol Biol. 1993 Dec 20;234(4):915–925. doi: 10.1006/jmbi.1993.1644. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Kwong A. D., Pan Z. Q., Hurwitz J. Studies on the activator 1 protein complex, an accessory factor for proliferating cell nuclear antigen-dependent DNA polymerase delta. J Biol Chem. 1991 Jan 5;266(1):594–602. [PubMed] [Google Scholar]
- Maki S., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. III. Distinctive processive polymerases reconstituted from purified subunits. J Biol Chem. 1988 May 15;263(14):6561–6569. [PubMed] [Google Scholar]
- Marchetti M. E., Smith C. A., Schaffer P. A. A temperature-sensitive mutation in a herpes simplex virus type 1 gene required for viral DNA synthesis maps to coordinates 0.609 through 0.614 in UL. J Virol. 1988 Mar;62(3):715–721. doi: 10.1128/jvi.62.3.715-721.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcy A. I., Hwang C. B., Ruffner K. L., Coen D. M. Engineered herpes simplex virus DNA polymerase point mutants: the most highly conserved region shared among alpha-like DNA polymerases is involved in substrate recognition. J Virol. 1990 Dec;64(12):5883–5890. doi: 10.1128/jvi.64.12.5883-5890.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcy A. I., Olivo P. D., Challberg M. D., Coen D. M. Enzymatic activities of overexpressed herpes simplex virus DNA polymerase purified from recombinant baculovirus-infected insect cells. Nucleic Acids Res. 1990 Mar 11;18(5):1207–1215. doi: 10.1093/nar/18.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marians K. J. Prokaryotic DNA replication. Annu Rev Biochem. 1992;61:673–719. doi: 10.1146/annurev.bi.61.070192.003325. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Korn D., Wang T. S. The evolutionary conservation of DNA polymerase alpha. Nucleic Acids Res. 1988 Aug 25;16(16):7961–7973. doi: 10.1093/nar/16.16.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan S. J., Barlam T. F., Crumpacker C. S., Parris D. S. Two regions of the herpes simplex virus type 1 UL42 protein are required for its functional interaction with the viral DNA polymerase. J Virol. 1993 Oct;67(10):5922–5931. doi: 10.1128/jvi.67.10.5922-5931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell M. E., Elias P., Lehman I. R. Processive replication of single-stranded DNA templates by the herpes simplex virus-induced DNA polymerase. J Biol Chem. 1987 Mar 25;262(9):4252–4259. [PubMed] [Google Scholar]
- Onrust R., Stukenberg P. T., O'Donnell M. Analysis of the ATPase subassembly which initiates processive DNA synthesis by DNA polymerase III holoenzyme. J Biol Chem. 1991 Nov 15;266(32):21681–21686. [PubMed] [Google Scholar]
- Pan Z. Q., Chen M., Hurwitz J. The subunits of activator 1 (replication factor C) carry out multiple functions essential for proliferating-cell nuclear antigen-dependent DNA synthesis. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):6–10. doi: 10.1073/pnas.90.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris D. S., Cross A., Haarr L., Orr A., Frame M. C., Murphy M., McGeoch D. J., Marsden H. S. Identification of the gene encoding the 65-kilodalton DNA-binding protein of herpes simplex virus type 1. J Virol. 1988 Mar;62(3):818–825. doi: 10.1128/jvi.62.3.818-825.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podust V. N., Georgaki A., Strack B., Hübscher U. Calf thymus RF-C as an essential component for DNA polymerase delta and epsilon holoenzymes function. Nucleic Acids Res. 1992 Aug 25;20(16):4159–4165. doi: 10.1093/nar/20.16.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. Nonstructural proteins of herpes simplex virus. I. Purification of the induced DNA polymerase. J Virol. 1977 Nov;24(2):618–626. doi: 10.1128/jvi.24.2.618-626.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Lewis R. B., Powell K. L. Identification of the herpes simplex virus DNA polymerase gene. Nature. 1977 Oct 13;269(5629):621–623. doi: 10.1038/269621a0. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. Temperature-sensitive mutants in two distinct complementation groups of herpes simplex virus type 1 specify thermolabile DNA polymerase. J Gen Virol. 1981 May;54(Pt 1):219–222. doi: 10.1099/0022-1317-54-1-219. [DOI] [PubMed] [Google Scholar]
- Stow N. D. Sequences at the C-terminus of the herpes simplex virus type 1 UL30 protein are dispensable for DNA polymerase activity but not for viral origin-dependent DNA replication. Nucleic Acids Res. 1993 Jan 11;21(1):87–92. doi: 10.1093/nar/21.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenberg P. T., Studwell-Vaughan P. S., O'Donnell M. Mechanism of the sliding beta-clamp of DNA polymerase III holoenzyme. J Biol Chem. 1991 Jun 15;266(17):11328–11334. [PubMed] [Google Scholar]
- Tabor S., Huber H. E., Richardson C. C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J Biol Chem. 1987 Nov 25;262(33):16212–16223. [PubMed] [Google Scholar]
- Tan C. K., Castillo C., So A. G., Downey K. M. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. J Biol Chem. 1986 Sep 15;261(26):12310–12316. [PubMed] [Google Scholar]
- Tenney D. J., Hurlburt W. W., Bifano M., Stevens J. T., Micheletti P. A., Hamatake R. K., Cordingley M. G. Deletions of the carboxy terminus of herpes simplex virus type 1 UL42 define a conserved amino-terminal functional domain. J Virol. 1993 Apr;67(4):1959–1966. doi: 10.1128/jvi.67.4.1959-1966.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney D. J., Micheletti P. A., Stevens J. T., Hamatake R. K., Matthews J. T., Sanchez A. R., Hurlburt W. W., Bifano M., Cordingley M. G. Mutations in the C terminus of herpes simplex virus type 1 DNA polymerase can affect binding and stimulation by its accessory protein UL42 without affecting basal polymerase activity. J Virol. 1993 Jan;67(1):543–547. doi: 10.1128/jvi.67.1.543-547.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Functions of replication factor C and proliferating-cell nuclear antigen: functional similarity of DNA polymerase accessory proteins from human cells and bacteriophage T4. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1023–1027. doi: 10.1073/pnas.87.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Replication factors required for SV40 DNA replication in vitro. I. DNA structure-specific recognition of a primer-template junction by eukaryotic DNA polymerases and their accessory proteins. J Biol Chem. 1991 Jan 25;266(3):1950–1960. [PubMed] [Google Scholar]
- Vaughan P. J., Purifoy D. J., Powell K. L. DNA-binding protein associated with herpes simplex virus DNA polymerase. J Virol. 1985 Feb;53(2):501–508. doi: 10.1128/jvi.53.2.501-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Carmichael E. P., Aschman D. P., Goldstein D. J., Schaffer P. A. Genetic and phenotypic characterization of mutants in four essential genes that map to the left half of HSV-1 UL DNA. Virology. 1987 Nov;161(1):198–210. doi: 10.1016/0042-6822(87)90186-3. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Lee K. J., Sabourin D. J., Schaffer P. A. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J Virol. 1983 Jan;45(1):354–366. doi: 10.1128/jvi.45.1.354-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. A., Nelson N. J., McGeoch D. J., Challberg M. D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988 Feb;62(2):435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Weller S. K. UL5, a protein required for HSV DNA synthesis: genetic analysis, overexpression in Escherichia coli, and generation of polyclonal antibodies. Virology. 1988 Oct;166(2):366–378. doi: 10.1016/0042-6822(88)90507-7. [DOI] [PubMed] [Google Scholar]