Abstract
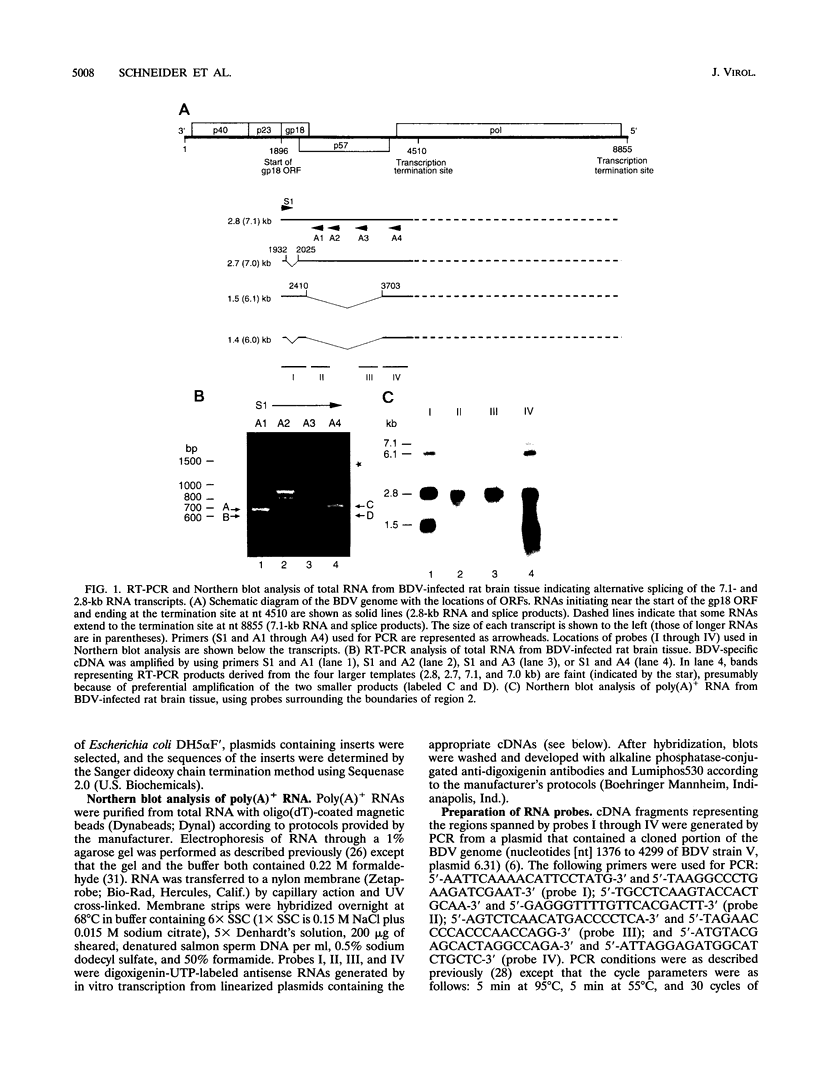
Borna disease virus (BDV) is a nonsegmented, negative-strand RNA virus related to rhabdoviruses and paramyxoviruses. Unlike animal viruses of these two families, BDV transcribes RNAs in the nuclei of infected cells and produces high levels of transcripts containing multiple open reading frames. Previous Northern blot analysis of RNA from BDV-infected rat brain tissue has shown that two viral transcripts, a 6.1-kb RNA and a 1.5-kb RNA, lack regions that are internal to two otherwise identical transcripts, the 7.1-kb RNA and the 2.8-kb RNA, respectively (T. Briese, A. Schneemann, A. Lewis, Y. Park, S. Kim, H. Ludwig, and W. I. Lipkin, Proc. Natl. Acad. Sci. USA 91:4362-4366, 1994). To determine the precise location of this deletion, we performed reverse transcription PCR analysis using total RNA from BDV-infected rat brain tissue. This investigation resulted in the identification of two introns in the 7.1- and 2.8-kb RNAs, which can be alternatively spliced to yield additional RNA species, including the 6.1- and 1.5-kb RNAs. Transient transfection of COS-7 cells with a cDNA clone of the 2.8-kb RNA resulted in the production of both the 2.8-kb RNA and the 1.5-kb RNA, confirming the theory that the 2.8-kb RNA is a sufficient substrate for splicing in mammalian cells. Splicing has not previously been observed in nonsegmented, negative-strand RNA viruses and presumably serves as a mechanism by which expression of BDV proteins is regulated in infected cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L., Riegel S., Ludwig H., Amsterdam J. D., Lange W., Koprowski H. Borna disease virus-specific antibodies in patients with HIV infection and with mental disorders. Lancet. 1988 Sep 17;2(8612):689–689. doi: 10.1016/s0140-6736(88)90505-3. [DOI] [PubMed] [Google Scholar]
- Bode L., Steinbach F., Ludwig H. A novel marker for Borna disease virus infection. Lancet. 1994 Jan 29;343(8892):297–298. doi: 10.1016/s0140-6736(94)91147-9. [DOI] [PubMed] [Google Scholar]
- Briese T., Schneemann A., Lewis A. J., Park Y. S., Kim S., Ludwig H., Lipkin W. I. Genomic organization of Borna disease virus. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T., de la Torre J. C., Lewis A., Ludwig H., Lipkin W. I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler A. J., Chang D. D., Graw S. L., Brook J. D., Haber D. A., Sharp P. A., Housman D. E. Exon amplification: a strategy to isolate mammalian genes based on RNA splicing. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4005–4009. doi: 10.1073/pnas.88.9.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone K. M., Rubin S. A., Sierra-Honigmann A. M., Lederman H. M. Characterization of a glial cell line persistently infected with borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993 Mar;67(3):1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R., Derse D. Translation of equine infectious anemia virus bicistronic tat-rev mRNA requires leaky ribosome scanning of the tat CTG initiation codon. J Virol. 1993 Mar;67(3):1433–1440. doi: 10.1128/jvi.67.3.1433-1440.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt B., Oldstone C., de la Torre J. C. Sequence and genome organization of Borna disease virus. J Virol. 1994 Mar;68(3):1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East J. L., Kingsbury D. W. Mumps virus replication in chick embryo lung cells: properties of ribonucleic acids in virions and infected cells. J Virol. 1971 Aug;8(2):161–173. doi: 10.1128/jvi.8.2.161-173.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z. F., Amsterdam J. D., Kao M., Shankar V., Koprowski H., Dietzschold B. Detection of Borna disease virus-reactive antibodies from patients with affective disorders by western immunoblot technique. J Affect Disord. 1993 Jan;27(1):61–68. doi: 10.1016/0165-0327(93)90098-5. [DOI] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Hirano N., Kao M., Ludwig H. Persistent, tolerant or subacute infection in Borna disease virus-infected rats. J Gen Virol. 1983 Jul;64(Pt 7):1521–1530. doi: 10.1099/0022-1317-64-7-1521. [DOI] [PubMed] [Google Scholar]
- Jackson I. J. A reappraisal of non-consensus mRNA splice sites. Nucleic Acids Res. 1991 Jul 25;19(14):3795–3798. doi: 10.1093/nar/19.14.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Lipkin W. I., Travis G. H., Carbone K. M., Wilson M. C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H., Bode L., Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- Marchuk D., Drumm M., Saulino A., Collins F. S. Construction of T-vectors, a rapid and general system for direct cloning of unmodified PCR products. Nucleic Acids Res. 1991 Mar 11;19(5):1154–1154. doi: 10.1093/nar/19.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Narayan O., Herzog S., Frese K., Scheefers H., Rott R. Behavioral disease in rats caused by immunopathological responses to persistent borna virus in the brain. Science. 1983 Jun 24;220(4604):1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. In vitro splicing of influenza viral NS1 mRNA and NS1-beta-globin chimeras: possible mechanisms for the control of viral mRNA splicing. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5444–5448. doi: 10.1073/pnas.83.15.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper J. M., Richt J. A., Brown L., Rott R., Narayan O., Clements J. E. Genomic organization of the structural proteins of borna disease virus revealed by a cDNA clone encoding the 38-kDa protein. Virology. 1993 Jul;195(1):229–238. doi: 10.1006/viro.1993.1364. [DOI] [PubMed] [Google Scholar]
- Rott R., Herzog S., Fleischer B., Winokur A., Amsterdam J., Dyson W., Koprowski H. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985 May 10;228(4700):755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- Schneider P. A., Briese T., Zimmermann W., Ludwig H., Lipkin W. I. Sequence conservation in field and experimental isolates of Borna disease virus. J Virol. 1994 Jan;68(1):63–68. doi: 10.1128/jvi.68.1.63-68.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Pavlakis G. N. Mechanism of translation of monocistronic and multicistronic human immunodeficiency virus type 1 mRNAs. Mol Cell Biol. 1992 Jan;12(1):207–219. doi: 10.1128/mcb.12.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierer J., Riehle H., Grebenstein O., Binz T., Herzog S., Thiedemann N., Stitz L., Rott R., Lottspeich F., Niemann H. The 24K protein of Borna disease virus. J Gen Virol. 1992 Feb;73(Pt 2):413–416. doi: 10.1099/0022-1317-73-2-413. [DOI] [PubMed] [Google Scholar]
- Tsang S. S., Yin X., Guzzo-Arkuran C., Jones V. S., Davison A. J. Loss of resolution in gel electrophoresis of RNA: a problem associated with the presence of formaldehyde gradients. Biotechniques. 1993 Mar;14(3):380–381. [PubMed] [Google Scholar]





