Abstract
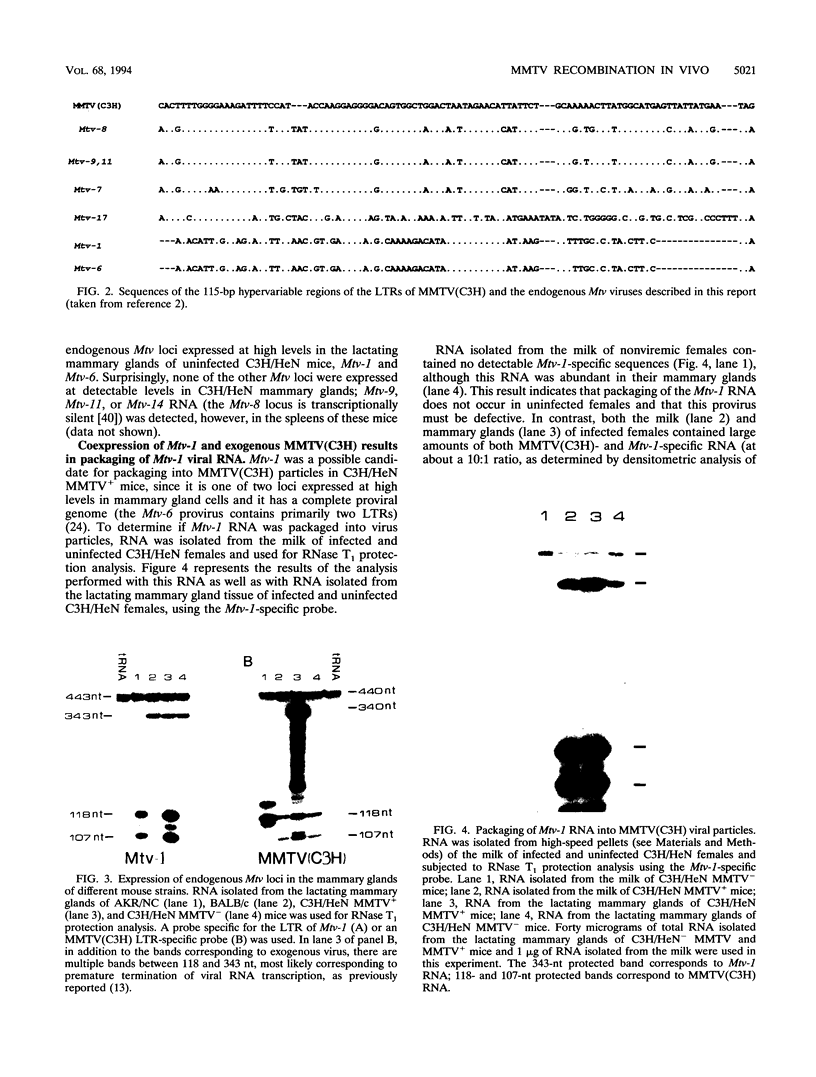
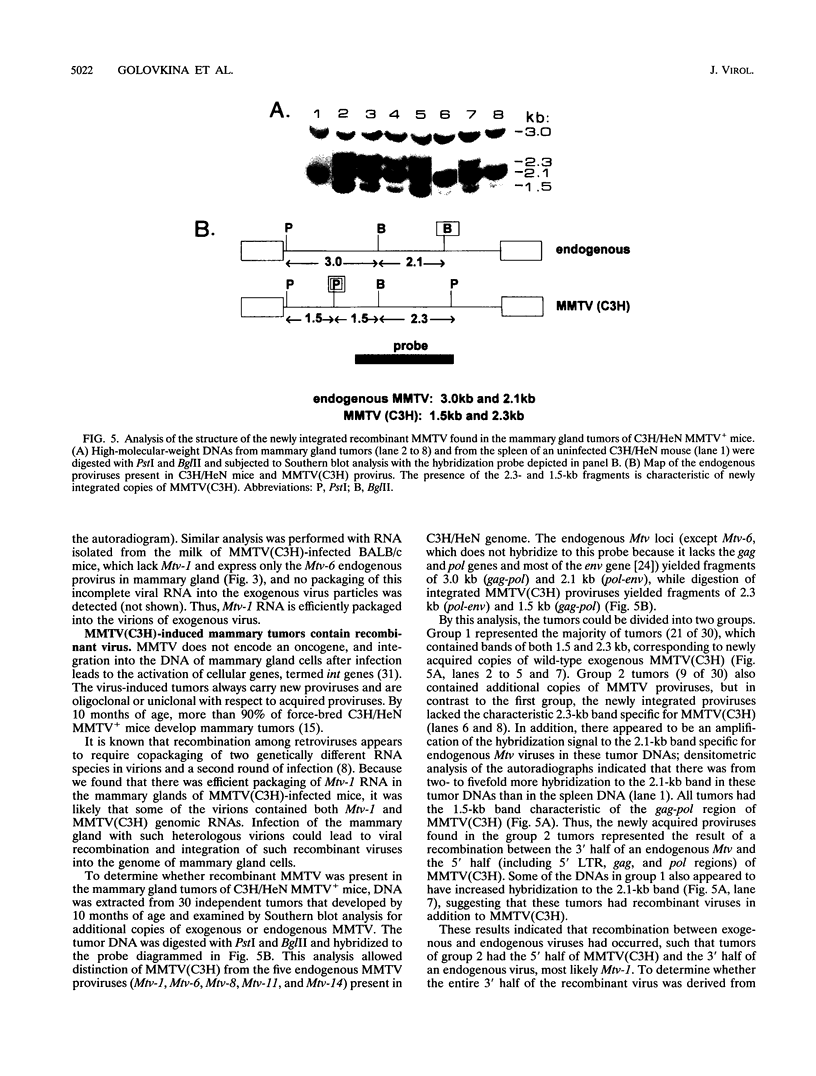
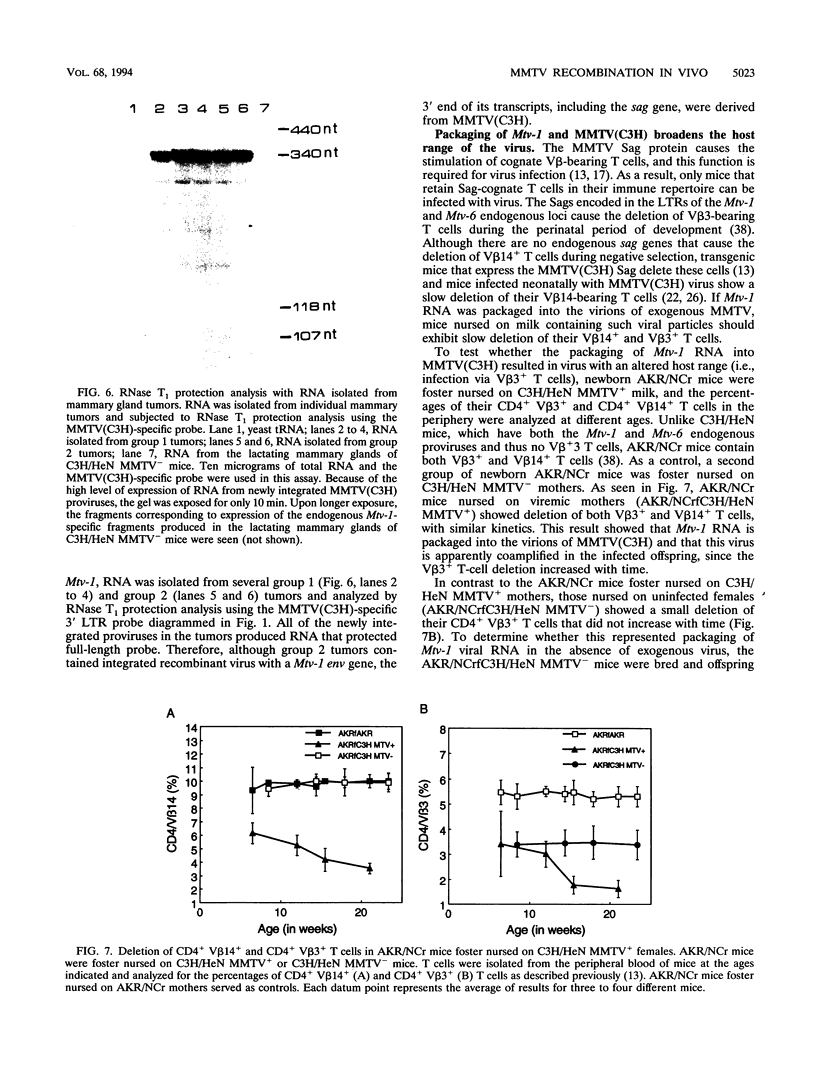
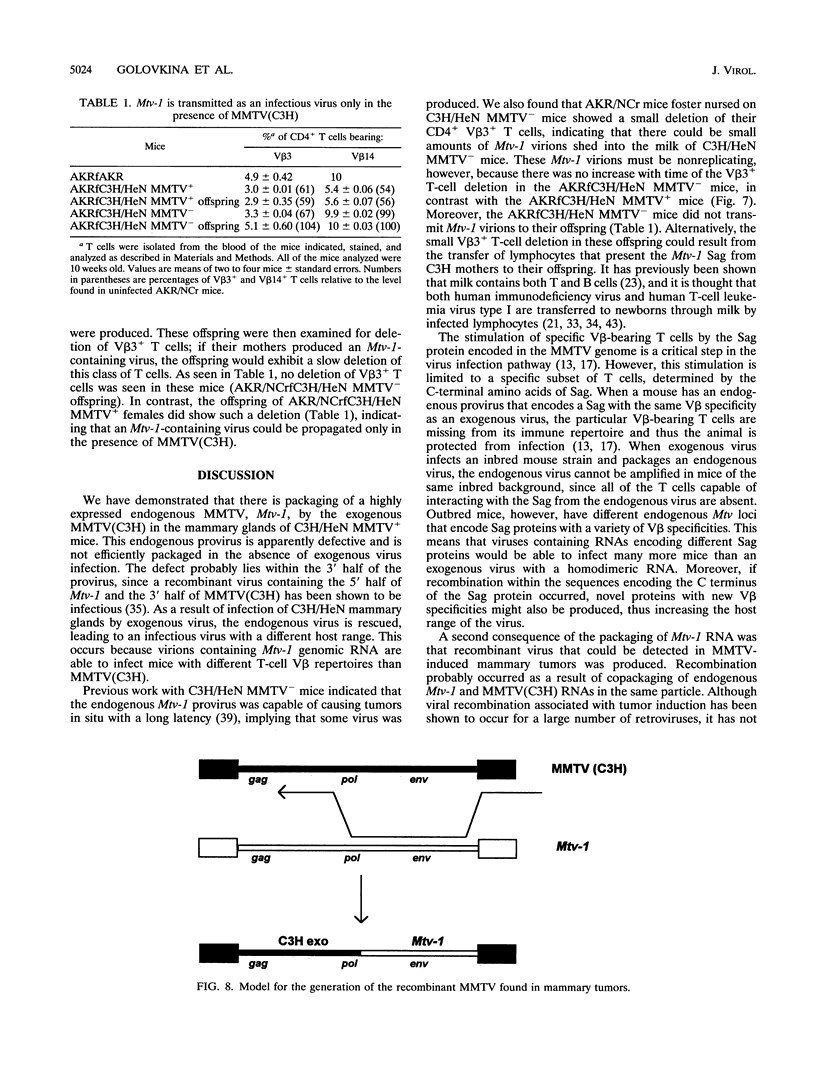
Mouse mammary tumor virus is a replication-competent B-type murine retrovirus responsible for mammary gland tumorigenesis in some strains of laboratory mice. Mouse mammary tumor virus is transmitted horizontally through the milk (exogenous or milk-borne virus) to susceptible offspring or vertically through the germ line (endogenous provirus). Exogenously acquired and some endogenous mouse mammary tumor viruses are expressed at high levels in lactating mammary glands. We show here that there is packaging of the endogenous Mtv-1 virus, which is expressed at high levels in the lactating mammary glands of C3H/HeN mice, by the virions of exogenous C3H mouse mammary tumor virus [MMTV(C3H)]. The mammary tumors induced in C3H/HeN mice infected with exogenous MMTV (C3H) virus contained integrated copies of recombinant virus containing a region of the env gene from an endogenous virus. This finding indicates that there was copackaging of the Mtv-1 and MMTV(C3H) RNAs in the same virions. Moreover, because Mtv-1 encodes a superantigen protein with a V beta specificity different from that encoded by the exogenous virus, the packaging of Mtv-1 results in an infectious virus with a broader host range than MMTV(C3H).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Shakhov A. N., Scarpellino L., Kolb E., Müller V., Vessaz-Shaw A., Fuchs R., Blöchlinger K., Rollini P., Billotte J. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991 Mar 21;350(6315):207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- Brandt-Carlson C., Butel J. S., Wheeler D. Phylogenetic and structural analyses of MMTV LTR ORF sequences of exogenous and endogenous origins. Virology. 1993 Mar;193(1):171–185. doi: 10.1006/viro.1993.1113. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Henrard D., Lee I., Ross S. R. The mouse mammary tumor virus long terminal repeat directs expression in epithelial and lymphoid cells of different tissues in transgenic mice. J Virol. 1987 Oct;61(10):3013–3019. doi: 10.1128/jvi.61.10.3013-3019.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Kappler J. W., Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991 Mar 21;350(6315):203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- Choi Y., Marrack P., Kappler J. W. Structural analysis of a mouse mammary tumor virus superantigen. J Exp Med. 1992 Mar 1;175(3):847–852. doi: 10.1084/jem.175.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel F., Hoggan M. D., Willey R. L., Strebel K., Martin M. A., Repaske R. Genetic recombination of human immunodeficiency virus. J Virol. 1989 Mar;63(3):1455–1459. doi: 10.1128/jvi.63.3.1455-1459.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse C. A., Pauley R. J. Molecular cloning and sequencing of the MTV-1 LTR: evidence for a LTR sequence alteration. Virus Res. 1989 Feb;12(2):123–137. doi: 10.1016/0168-1702(89)90059-2. [DOI] [PubMed] [Google Scholar]
- DiFronzo N. L., Holland C. A. A direct demonstration of recombination between an injected virus and endogenous viral sequences, resulting in the generation of mink cell focus-inducing viruses in AKR mice. J Virol. 1993 Jul;67(7):3763–3770. doi: 10.1128/jvi.67.7.3763-3770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson P. J., Knight A. M., Fairchild S., Simpson E., Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991 Feb 7;349(6309):531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- Frankel W. N., Rudy C., Coffin J. M., Huber B. T. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature. 1991 Feb 7;349(6309):526–528. doi: 10.1038/349526a0. [DOI] [PubMed] [Google Scholar]
- Golovkina T. V., Chervonsky A., Dudley J. P., Ross S. R. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell. 1992 May 15;69(4):637–645. doi: 10.1016/0092-8674(92)90227-4. [DOI] [PubMed] [Google Scholar]
- Golovkina T. V., Chervonsky A., Prescott J. A., Janeway C. A., Jr, Ross S. R. The mouse mammary tumor virus envelope gene product is required for superantigen presentation to T cells. J Exp Med. 1994 Feb 1;179(2):439–446. doi: 10.1084/jem.179.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovkina T. V., Prescott J. A., Ross S. R. Mouse mammary tumor virus-induced tumorigenesis in sag transgenic mice: a laboratory model of natural selection. J Virol. 1993 Dec;67(12):7690–7694. doi: 10.1128/jvi.67.12.7690-7694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Duesberg P. H. Retroviral recombination during reverse transcription. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2052–2056. doi: 10.1073/pnas.87.6.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W., Waanders G. A., Shakhov A. N., Scarpellino L., Acha-Orbea H., MacDonald H. R. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell. 1993 Aug 13;74(3):529–540. doi: 10.1016/0092-8674(93)80054-i. [DOI] [PubMed] [Google Scholar]
- Henrard D., Ross S. R. Endogenous mouse mammary tumor virus is expressed in several organs in addition to the lactating mammary gland. J Virol. 1988 Aug;62(8):3046–3049. doi: 10.1128/jvi.62.8.3046-3049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1556–1560. doi: 10.1073/pnas.87.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Retroviral recombination and reverse transcription. Science. 1990 Nov 30;250(4985):1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- Ichimaru M., Ikeda S., Kinoshita K., Hino S., Tsuji Y. Mother-to-child transmission of HTLV-1. Cancer Detect Prev. 1991;15(3):177–181. [PubMed] [Google Scholar]
- Ignatowicz L., Kappler J., Marrack P. The effects of chronic infection with a superantigen-producing virus. J Exp Med. 1992 Apr 1;175(4):917–923. doi: 10.1084/jem.175.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain L., Vidyasagar D., Xanthou M., Ghai V., Shimada S., Blend M. In vivo distribution of human milk leucocytes after ingestion by newborn baboons. Arch Dis Child. 1989 Jul;64(7 Spec No):930–933. doi: 10.1136/adc.64.7_spec_no.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C., Peters G., Pauley R., Morris V., Michalides R., Dudley J., Green M., Davisson M., Prakash O., Vaidya A. A standardized nomenclature for endogenous mouse mammary tumor viruses. J Virol. 1987 May;61(5):1651–1654. doi: 10.1128/jvi.61.5.1651-1654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao N. S., Maltzman J., Raulet D. H. Positive selection determines T cell receptor V beta 14 gene usage by CD8+ T cells. J Exp Med. 1989 Jul 1;170(1):135–143. doi: 10.1084/jem.170.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature. 1991 Feb 7;349(6309):524–526. doi: 10.1038/349524a0. [DOI] [PubMed] [Google Scholar]
- Mok E., Golovkina T. V., Ross S. R. A mouse mammary tumor virus mammary gland enhancer confers tissue-specific but not lactation-dependent expression in transgenic mice. J Virol. 1992 Dec;66(12):7529–7532. doi: 10.1128/jvi.66.12.7529-7532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Pandey R., Ghosh A. K., Kumar D. V., Bachman B. A., Shibata D., Roy-Burman P. Recombination between feline leukemia virus subgroup B or C and endogenous env elements alters the in vitro biological activities of the viruses. J Virol. 1991 Dec;65(12):6495–6508. doi: 10.1128/jvi.65.12.6495-6508.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Choi Y., Kushnir E., Kappler J., Marrack P. The open reading frames in the 3' long terminal repeats of several mouse mammary tumor virus integrants encode V beta 3-specific superantigens. J Exp Med. 1992 Jan 1;175(1):41–47. doi: 10.1084/jem.175.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji F., Ohashi K., Tokugawa Y., Kamiura S., Azuma C., Tanizawa O. Perinatal infection of human T-lymphotropic virus type I, the etiologic virus of adult T-cell leukemia/lymphoma. DNA amplification of specific human T-lymphotropic virus type I sequences. Cancer. 1990 Nov 1;66(9):1933–1937. doi: 10.1002/1097-0142(19901101)66:9<1933::aid-cncr2820660914>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Seltzer V., Benjamin F. Breast-feeding and the potential for human immunodeficiency virus transmission. Obstet Gynecol. 1990 Apr;75(4):713–715. [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Construction of a clonable, infectious, and tumorigenic mouse mammary tumor virus provirus and a derivative genetic vector. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9655–9659. doi: 10.1073/pnas.85.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Moroni C., Coffin J. M. Virological events leading to spontaneous AKR thymomas. J Virol. 1991 Mar;65(3):1273–1285. doi: 10.1128/jvi.65.3.1273-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhlmann H., Berg P. Homologous recombination of copackaged retrovirus RNAs during reverse transcription. J Virol. 1992 Apr;66(4):2378–2388. doi: 10.1128/jvi.66.4.2378-2388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonari K., Fairchild S., Rosenwasser O. A. Influence of viral superantigens on V beta- and V alpha-specific positive and negative selection. Immunol Rev. 1993 Feb;131:131–168. doi: 10.1111/j.1600-065x.1993.tb01534.x. [DOI] [PubMed] [Google Scholar]
- Woodland D. L., Happ M. P., Gollob K. J., Palmer E. An endogenous retrovirus mediating deletion of alpha beta T cells? Nature. 1991 Feb 7;349(6309):529–530. doi: 10.1038/349529a0. [DOI] [PubMed] [Google Scholar]
- Yang J. N., Dudley J. Endogenous Mtv-8 or a closely linked sequence stimulates rearrangement of the downstream V kappa 9 gene. J Immunol. 1992 Aug 15;149(4):1242–1251. [PubMed] [Google Scholar]
- Yoshimura F. K., Davison B., Chaffin K. Murine leukemia virus long terminal repeat sequences can enhance gene activity in a cell-type-specific manner. Mol Cell Biol. 1985 Oct;5(10):2832–2835. doi: 10.1128/mcb.5.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J. B., Cooper D. A., Johnson R. O., Gold J. Postnatal transmission of AIDS-associated retrovirus from mother to infant. Lancet. 1985 Apr 20;1(8434):896–898. doi: 10.1016/s0140-6736(85)91673-3. [DOI] [PubMed] [Google Scholar]
- van Nie R., Verstraeten A. A. Studies of genetic transmission of mammary tumour virus by C3Hf mice. Int J Cancer. 1975 Dec 15;16(6):922–931. doi: 10.1002/ijc.2910160606. [DOI] [PubMed] [Google Scholar]