Abstract
Allogeneic stem cell transplantation with reduced intensity conditioning (RIC SCT) has the potential to lead to long-term remissions for patients with lymphoma. However, the role of RIC SCT in the treatment of lymphoma is still unclear. Specifically, the relative benefit of RIC SCT across lymphoma histologies and the prognostic factors in this population are incompletely defined. We retrospectively analyzed the outcomes of 87 patients with advanced lymphoma who underwent RIC SCT at Dana-Farber Cancer Institute over a 6-year period with a homogeneous conditioning regimen consisting of fludarabine and low-dose busulfan. Thirty-six patients had Hodgkin disease (HD) and 51 had non-Hodgkin lymphoma (NHL). Sixty-eight percent had undergone prior autologous transplantation. The 1-year cumulative incidence of non-relapse mortality was 13%, and the 3-year cumulative incidence of progression was 49%. The incidence of grade 3–4 acute GVHD was 11%. The 2-year cumulative incidence of chronic GVHD was 68%, and its development was associated with a decreased risk of progression and an improved progression-free survival. Three-year overall survival (OS) was 56% for patients with HD, 81% for indolent NHL, 42% for aggressive NHL, and 40% for mantle cell lymphoma. The corresponding figures for 3-year PFS were 22%, 59%, 22%, and 30%, respectively. Multivariate analysis identified elevated pre-transplantation LDH as an adverse factor for PFS, while indolent NHL histology was favorable. For OS, advanced age and elevated pre-transplantation LDH were adverse factors, while indolent NHL histology was favorable. Low early donor chimerism was not predictive of poor outcome in univariate or multivariate analyses. Moreover, progression was not associated with loss of chimerism. These results emphasize the importance of lymphoma histology for patients undergoing RIC SCT, as well as the lack of relevance of donor chimerism for outcome in this patient population.
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (SCT) carries the theoretical promise of cure for patients with lymphoproliferative disease by relying on a graft-versus-lymphoma (GVL) effect. However, lymphomas represent a heterogeneous group of diseases that differ in their ability to be cured with standard or high-dose chemotherapy, as well as in their susceptibility to GVL1. In the SCT experience with lymphoma using myeloablative conditioning regimens, the decreased risk of relapse compared to autologous transplantation was offset by a high treatment-related mortality (TRM), such that there was no clear benefit of SCT over autologous transplantation 2,3. There was also the suggestion that the benefit of allogeneic transplantation was in avoiding tumor contamination of the graft4. The use of reduced intensity conditioned stem cell transplantation (RIC SCT) led to a substantial decrease in the incidence of TRM, apparently without a commensurate increase in the risk of relapse5, emphasizing the importance of the GVL effect. Nevertheless, the optimal role and timing of RIC SCT in lymphoma therapy are still ill-defined, and vary by lymphoma subtype. At one end of the spectrum are lymphomas such as follicular lymphoma (FL), where SCT carries the best chance of long-term cure, but at the cost of a significantly higher morbidity and mortality than standard treatment for a disease that may be clinically indolent. At the other end of the spectrum are lymphomas such as Burkitt’s lymphoma which seem to be minimally susceptible to GVL and hence are by and large incurable with RIC SCT.
The published data on RIC SCT for lymphomas is mostly made up of single-institution case series that focus on one particular histology. Here we report our institution’s experience with RIC SCT using a uniform conditioning regimen applied to 87 patients with Hodgkin disease (HD) or B-cell non-Hodgkin lymphomas (NHL), with the objectives of comparing outcomes among different lymphoma subgroups, extracting risk factors for progression and survival, and examining the relevance of donor chimerism in this setting.
METHODS
Patients
We reviewed the medical records of all consecutive adult patients with HD or NHL (restricted to indolent B-cell NHL, aggressive B-cell NHL, or mantle cell lymphoma) who underwent first allogeneic SCT with reduced intensity conditioning at the combined Dana-Farber/Brigham and Women’s Hospital transplant program between July 2000 and July 2006. Patients receiving umbilical cord grafts were not included in this study. Patients with chronic lymphocytic leukemia/small lymphocytic leukemia were excluded, as their outcomes have been previously reported6. 87 patients met the above criteria. Informed consent was obtained under an IRB-approved protocol, and this study was conducted in accordance with the principles of the Declaration of Helsinki.
Transplantation
Patients were conditioned using a non-myeloablative regimen consisting of busulfan (0.8 mg/kg/d intravenously for 4 days) and fludarabine (30 mg/m2/d intravenously for 4 days), on days −5 to −2. They received stem cells from peripheral blood (PB) for all but 2 patients (who received bone marrow) on day 0. For patients receiving PB stem cells, the median dose was 7.5 106 CD34+ cells/kg (range, 2.3–23.3). Most patients received a graft-versus-host disease (GVHD) prophylaxis regimen consisting of tacrolimus + sirolimus +/− low-dose methotrexate. Acute GVHD was graded according to the modified Glucksberg scale7,8. Supportive care for all patients consisted of Pneumocystis jiroveci prophylaxis and varicella zoster virus/herpes simplex virus prophylaxis. Viral load monitoring was performed for cytomegalovirus (CMV), with preemptive treatment in cases of reactivation.
Chimerism analysis
Donor-derived hematopoiesis was assessed after SCT on unfractionated bone marrow aspirates or PB on approximately day +30 (range 20–50) and day +100 (range 75–120). A third sample was obtained 4–7 months after transplantation when possible. Genotype of donor and recipient were determined using DNA obtained from pre-transplantation samples. Nine short tandem repeat (STR) loci were typed using the ABI Profiler Plus Kit (Applied Biosystems Inc) and the ABI 310 or ABI 3130 Genetic Analyzer. Informative alleles unique to donor or recipient were used in the determination of post-transplantation chimerism.
Statistics
Overall survival and disease-free survival were calculated using the Kaplan-Meier method. Overall survival was defined as the time from stem cell infusion to death from any cause. Patients who were alive or lost to follow-up were censored at the time of last follow-up. Progression-free survival was defined as the time from stem cell infusion to progression or death from any cause. Patients who were alive without progression were censored at the time last seen alive and progression-free. The log-rank test was used for comparisons of Kaplan-Meier curves. Cumulative incidence curves for non-relapse death and progression with or without death were constructed considering time to progression and time to non-relapse death as competing risks. Competing risks analysis was also used to determine the cumulative incidence of graft-versus-host disease (GVHD), considering death without GVHD as a competing risk. The difference between cumulative incidence curves in the presence of a competing risk was tested using the Gray method9. Potential prognostic factors for survival, disease-free survival, progression, and non-relapse death were examined in the proportional hazards model as well as in the competing risks regression model10. The impact of GVHD on outcome was examined using proportional hazards model with GVHD as a time-dependent variable. Interaction terms including interaction with time were examined in the proportional hazards regression model. Proportional hazards assumption for each variable of interest was tested.
RESULTS
Patient characteristics
The baseline demographic and clinical characteristics of the 87 patients in this study are listed in Table 1. Among them, 36 had HD (all of whom had classical HD) and 51 had NHL. Of the patients with NHL, 13 (26%) had indolent lymphoma, 23 (45%) had aggressive lymphoma, and 15 (29%) had mantle cell lymphoma. The median age for the cohort was 46 (range, 18–64). Twenty-eight percent of the patients were transplanted in complete remission (CR), and 49% in partial remission (PR); 20% had stable or progressive disease after their last therapy, and the remaining 3% had untreated relapse. The median number of prior lines of therapy was 4 (range, 1–10). Sixty-eight percent of the patients had received a prior autograft (94% of patients with HD, 15% with indolent NHL, 78% with aggressive NHL, and 33% with mantle cell lymphoma). Thirty-eight percent of patients were transplanted from matched related, 56% from matched unrelated, and 6% from mismatched donors.
Table 1.
Baseline characteristics of the patients
| Variable | Non-Hodgkin Lymphoma Number, no. (%*) | Hodgkin Disease Number, no. (%*) |
|---|---|---|
| Number of patients: | 51 | 36 |
| Age in years (median, range) | 51 (34–64) | 31 (18–50) |
| Histology: | ||
| Classical Hodgkin | 36 (100) | |
| Indolent B-NHL | 13 (26) | |
| Follicular | 12 (24) | |
| Marginal zone | 1 (2) | |
| Aggressive B-NHL | 23 (45) | |
| Diffuse large B cell | 10 (20) | |
| Transformed follicular | 13 (25) | |
| Mantle cell | 15 (29) | |
| Disease status at transplantation: | ||
| CR | 14 (27) | 10 (28) |
| PR | 25 (49) | 18 (50) |
| SD | 6 (12) | 2 (6) |
| PD | 3 (6) | 6 (17) |
| Untreated | 3 (6) | 0 (0) |
| Marrow involvement at transplantation: | ||
| Yes | 7 (14) | 0 (0) |
| No | 27 (53) | 9 (25) |
| Unknown | 17 (33) | 27 (75) |
| Best response to chemotherapy: | ||
| CR | 39 (76) | 33 (92) |
| PR | 12 (24) | 3 (8) |
| Chemosensitivity at transplantation: | ||
| Yes | 42 (82) | 28 (78) |
| No | 5 (10) | 4 (11) |
| Untreated relapse | 4 (8) | 4 (11) |
| Number of prior therapies (median, range): | 4 (1–10) | 4 (2–8) |
| Prior autologous transplantation | ||
| Yes | 25 (49) | 34 (94) |
| No | 26 (51) | 2 (6) |
| Time from diagnosis to SCT, years (median, range): | 4 (0–16) | 3 (1–25) |
| LDH pre-transplantation: | ||
| Normal | 46 (90) | 32 (92) |
| Elevated | 5 (10) | 3 (8) |
| Graft source: | ||
| Peripheral blood | 50 (98) | 35 (97) |
| Bone marrow | 1 (2) | 1 (3) |
| CD34 dose (median, range)†: | 8.1 (2.4–23.3) | 7.2 (2.3–21.6) |
| Match: | ||
| MRD | 22 (43) | 11 (31) |
| MUD | 27 (53) | 22 (61) |
| Mismatched related | 1 (2) | 0 (0) |
| Mismatched unrelated | 1 (2) | 3 (8) |
| Gender match: | ||
| Female -> Male | 16 (31) | 6 (17) |
| GVHD prophylaxis: | ||
| CnI +/− steroids | 14 (27) | 3 (8) |
| CnI + Mtx | 5 (10) | 9 (25) |
| CnI + Siro +/− Mtx | 32 (63) | 24 (67) |
| Recipient CMV seropositive: | 17 (33) | 10 (28) |
| Donor CMV seropositive: | 17 (33) | 9 (25) |
CR indicates complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; SCT, allogeneic hematopoietic stem cell transplantation; MRD, matched related donor; MUD, matched unrelated donor; GVHD, graft-versus-host disease; CnI, calcineurin inhibitor (cyclosporine or tacrolimus); Mtx, methotrexate; Siro, sirolimus; and CMV, cytomegalovirus
Percentages may not add to 100 because of rounding
Cell dose unit is 106 per kilogram of recipient weight
Engraftment
Thirty-six patients had an ANC nadir below 500 cells/µl, and 25% a platelet nadir below 20,000 cells/µl. All patients engrafted, with a median time to neutrophil recovery of 13 days (range, 1–21), and a median time to platelet recovery of 20 days (range, 3–53). The time to neutrophil or platelet recovery was not significant affected by the lymphoma histology or the CD34 cell dose.
Graft-versus-host disease
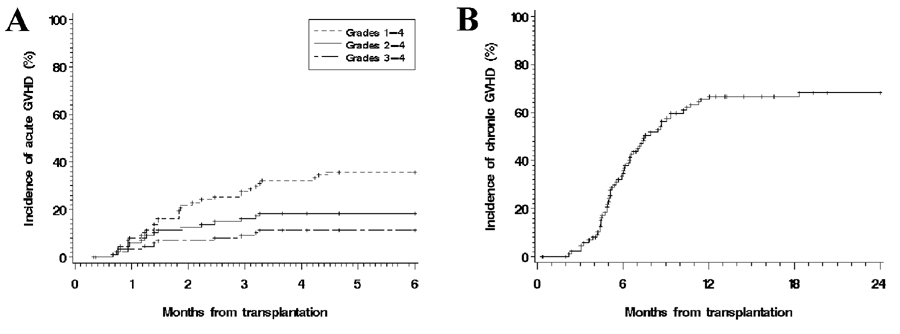
The cumulative incidences of acute and chronic GVHD are shown in Figure 1a and Figure 1b, respectively. The 100-day incidence of acute GVHD was 18% for grades 2–4 and 11% for grade 3–4. The cumulative incidence of grade 3–4 acute GVHD at 100 days was 12% for matched related donors, 10% for matched unrelated donors, and 20% for mismatched donors (p=0.8). The 2-year incidence of chronic GVHD was 68% (limited, 8%; extensive, 60%). When analyzed by histology, the 100-day incidences of grade 2–4 acute GVHD were 22%, 8%, 17%, and 20% for Hodgkin, indolent, aggressive, and mantle cell histologies, respectively (p=0.5). The corresponding 2-year incidences of chronic GVHD were 67%, 69%, 78%, and 53%, respectively (p=0.5). In competing risks regression analyses, only the use of sirolimus in the prophylaxis regimen was protective for grade 2–4 acute GVHD (HR=0.3 compared to calcineurin inhibitor + methotrexate, p=0.037). In analyses for chronic GVHD, only the use of a calcineurin inhibitor without methotrexate or sirolimus (HR compared to calcineurin inhibitor + methotrexate 2.4, p=0.036) was significant.
Figure 1. Cumulative incidence of acute and chronic graft-versus-host disease (GVHD).
(A) Acute GVHD; (B) Chronic GVHD.
The development of GVHD had a significant effect on outcomes. In proportional hazards analyses with GVHD added as a time-dependent covariate, grade 2–4 acute GVHD was associated with a significantly inferior PFS (HR=2.6, p=0.003) and inferior OS (HR=4.2, p<0.0001). Chronic GVHD was associated with improved PFS (HR=0.4, p=0.004), but no significant effect on OS. We examined interactions between GVHD and disease histology. Although the small numbers precluded definitive conclusions, the protective effect of chronic GVHD on PFS appeared to apply to all histologies except aggressive B-NHL.
Progression and non-relapse mortality
The 3-year cumulative incidence of progression (CIP) for all patients was 49% (95% confidence interval (CI), 37–60%) and non-relapse mortality (NRM) was 23% (CI, 12–33) (Table 2 and Figure 2). In competing risks univariate modeling, the non-GVHD factors associated with an increased incidence of progression were number of prior therapies (HR for >4 prior therapies 2.6, p=0.042) and elevated pre-transplantation LDH (HR=2.7, p=0.032). The only factor significantly associated with NRM was indolent NHL histology (HR=0, p not calculable). Although the 3-year NRM for patients with HD appeared lower than for patients with non-indolent NHL (15% vs 38%, respectively), this difference was not statistically significant (p=0.13). Elevated LDH and indolent histology remained significant in multivariable analyses (Table 3).
Table 2.
One-year and 3-year outcomes by histology
| Histology | Outcome | 1-year | 3-year |
|---|---|---|---|
| PFS | 53% (42–63) | 29% (17–40) | |
| All patients | OS | 76% (67–85) | 53% (41–66) |
| CIP | 34% (24–45) | 49% (37–60) | |
| NRM | 13% (6–20) | 23% (12–33) | |
| PFS | 77% (54–100) | 59% (31–87) | |
| Indolent NHL | OS | 92% (78–100) | 81% (56–100) |
| CIP | 23% (0–47) | 41% (11–70) | |
| NRM | 0% (NC) | 0% (NC) | |
| PFS | 48% (27–68) | 22% (0–45) | |
| Aggressive NHL | OS | 69% (50–88) | 42% (15–70) |
| CIP | 35% (15–55) | 40% (19–60) | |
| NRM | 17% (1–33) | 39% (9–69) | |
| PFS | 40% (15–65) | 30% (5–55) | |
| Mantle cell NHL | OS | 60% (35–85) | 40% (12–68) |
| CIP | 33% (8–58) | 33% (8–58) | |
| NRM | 27% (3–50) | 37% (8–65) | |
| PFS | 53% (36–69) | 22% (7–37) | |
| Hodgkin | OS | 81% (68–93) | 56% (38–74) |
| CIP | 39% (23–55) | 63% (45–81) | |
| NRM | 8% (0–18) | 15% (2–28) | |
PFS indicates progression-free survival; OS, overall survival; CIP, cumulative incidence of progression; NRM, cumulative incidence of non-progression mortality; NHL, non-Hodgkin lymphoma; NC, not calculable. Values in parentheses are 95% confidence intervals.
Figure 2. Progression and non-relapse mortality, stratified by histology.
(A) Cumulative incidence of progression; (B) Cumulative incidence of non-relapse mortality.
Table 3.
Summary of multivariate analyses
Survival
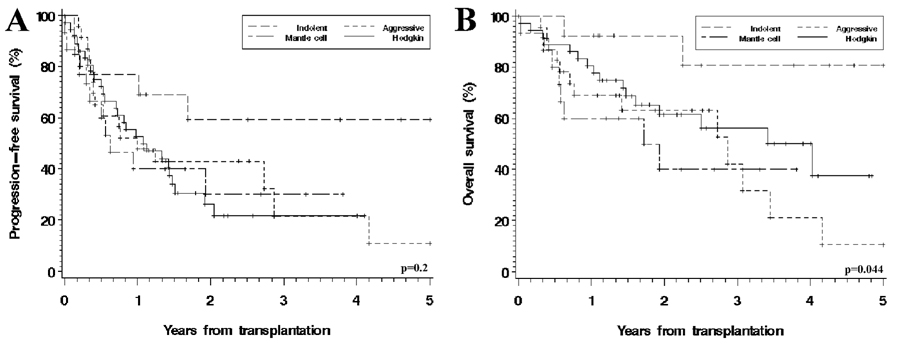
Median follow-up for survivors was 26 months (range, 7–73). The median follow-up was the same for NHL and HD patients. For the entire cohort, the 3-year progression-free survival (PFS) was 29% (CI, 17–40%), and overall survival (OS) was 53% (CI, 41–66%) (Table 2 and Figure 3).
Figure 3. Progression-free and overall survival, stratified by histology.
(A) Progression-free survival; (B) Overall survival.
We examined all baseline characteristics (Table 1) for their possible association with progression-free and overall survival in both univariate and multivariate analyses. HD, aggressive B-NHL and MCL, which had similar outcomes in univariate analysis, were collapsed into one group in the models to satisfy the proportional hazards assumption. The factors that were significantly associated with PFS in univariate analyses were elevated pre-transplant LDH (HR 4.2, p=0.0004), acute GVHD grades 3–4 (HR=4.1, p<0.0001), chronic GVHD (HR=0.4, p=0.002) and disease histology. The HR for PFS for indolent NHL, compared to other histologies, was 0.4 (p=0.045). Indolent histology and elevated LDH remained associated with PFS in multivariate modeling (see Table 3). In those models, we did not include GVHD since we included GVHD risk factors including match and prophylaxis regimen. For OS, the factors that were significant in univariate modeling were indolent histology (HR=0.2, p=0.027), use of sirolimus in the GVHD prophylaxis regimen (HR compared to calcineurin inhibitor + methotrexate=0.4, p=0.041), elevated pre-transplant LDH (HR=3.9, p=0.001), and acute GVHD grades 3–4 (HR=9.0, p<0.0001). In multivariate modeling, age over 50, elevated LDH and indolent NHL histology were significant (see Table 3).
Response to Donor Lymphocyte Infusions (DLI)
Of the 40 patients with disease relapse or progression, 18 received DLI (among whom 2 received DLI with expanded donor dendritic cells on a clinical protocol). The indication in all cases was disease progression or relapse. Three of those patients received DLI as consolidation after chemotherapy. There were 2 complete remissions (CR) and 2 partial remissions (PR), for an overall response rate of 22%. The response rate after DLI for patients with Hodgkin disease was 15% (2/13, both CRs), and for patients with aggressive NHL, 40% (2/5, both PRs). The median duration of response for those 4 patients was 11 months (range, 4–35 months). Three of the 18 patients treated with DLI (17%) are alive without evidence of disease relapse, at 4, 11 and 35 months after DLI. No patient with indolent or mantle cell lymphoma received DLI in this cohort. One patient with diffuse large B cell lymphoma (DLBCL) received DLI for secondary graft failure without evidence of relapse, but the patient expired 2 months later.
Relationship of chimerism with outcome
Among the 87 patients, 70 (80%) had a chimerism value available between days 20 and 50 after SCT, from PB or marrow. The concordance between PB and marrow chimerism values in 17 pairs of simultaneously drawn specimens was excellent, as previously described6; we therefore used whichever was available in the analyses below. Of the 17 patients without an available chimerism study, 2 died early, and the remainder had their first value drawn after day 50.
The median day 20–50 chimerism was 97% (range, 46–100), and was similar for patients with HD (median value 98%) and patients with NHL (median 96%). The day 20–50 chimerism had no significant association with PFS or OS, no matter where the cutoff value was chosen. Neither was the chimerism value associated with any significant effect when it was added to the multivariable models for PFS, OS, NRM, or CIP (data not shown). This remained true when HD and NHL were considered separately.
We also examined the relevance of chimerism information at the time of progression. Among the 40 patients with disease relapse or progression, 21 (52%) had an available chimerism within 1 month of relapse. The median value was 100%, and 95% of the chimerism values were ≥90%. Twenty-nine of the 40 relapsed patients (73%) had a chimerism study drawn within 2 months of relapse or progression. The median value was 100%, and 93% of the chimerism values were ≥90%. This held true for both patients with HD and those with NHL. Thus, progression was not associated with a loss of chimerism. Finally, chimerism had no bearing on the result of DLI therapy. Median chimerism pre-DLI was 100% for the 4 patients with response to DLI (range, 100–100) as well as for the 14 patients with no response to DLI (range, 93–100).
DISCUSSION
Our results confirm that RIC SCT has the potential to induce long-term remissions in patients with a variety of lymphomas. Our results are based on a retrospective analysis of a limited and hetereogenous sample of patients, which must be kept in mind when interpreting the results. Nonetheless, the inclusion of all lymphoma histologies in a single series with uniform conditioning regimen and the use of multivariable analysis to adjust for variables such as receipt of a prior autograft lead us to conclude that the long-term remission rates after SCT vary significantly across histologies, with a clearly superior outcome for indolent histologies.
Many of the patients in our cohort received non-standard GVHD prophylaxis regimens containing sirolimus, which appeared to have an influence GVHD rates. We are currently analyzing in more details the effect of sirolimus on transplantation outcome in this patient population. The use of multivariable analysis in a study of this size could certainly fail to uncover significant differences between GVHD prophylaxis regimens or other baseline factors, which may only be apparent in larger studies.
Patients with Hodgkin disease in our series had a low rate of PFS (mostly due to a high rate of progression), with a large difference between PFS and OS. This is consistent with other groups’ experiences23. As was the case for patients with aggressive lymphoma, most (94%) patients with HD in our study had failed a prior autologous transplantation, implying very aggressive disease. Yet there is in our series some evidence of a GVL effect (as suggested by the protective effect of chronic GVHD and by the ability to achieve durable responses with DLI), which has also been previously reported24–26. Intensifying the conditioning regimen before allogeneic transplantation has not been shown to be beneficial in this disease24–26; the challenge may therefore be to harness the GVL effect in time to prevent progression without relying on conditioning intensity to lengthen post-transplantation remission. Immune manipulations that target the interaction between the tumor cells and the immune system after transplantation may be a reasonable strategy in this setting.
The survival rate for indolent NHL patients in our series is comparable to previously published results5,11–15. The better outcome of those patients is not simply a function of a more indolent behavior after progression, since both PFS and OS were superior for patients with indolent NHL in multivariable analyses. It is also unlikely that this reflects a longer time to progression for indolent NHL, given how heavily pre-treated our patients were, with a median time to last relapse of 5.5 months. The trend towards a lower PFS with heavier pre-treatment, combined with the very favorable NRM, suggest that RIC SCT may warrant consideration earlier in the treatment course of patients with relapsed indolent NHL.
In our series, patients with aggressive NHL had a low rate of PFS, which resulted from both a high risk of non-relapse mortality and a high risk of progression. Prior studies have reported progression- or event-free survival close to or slightly higher than ours5,11,14,16–18. Comparative studies have also shown that patients with aggressive NHL do not do as well after RIC SCT as patients with indolent NHL5,11,17. In our series, as in the study of Rezvani and colleagues15, patients with transformed indolent lymphoma had a worse outcome than patients with non-transformed indolent lymphoma, comparable to that of patients with de novo aggressive histologies. The poorer outcome of patients with aggressive NHL occurred despite the fact that those patients were not more likely to have chemorefractory disease in our cohort. Our results suggest that aggressive NHL histologies may be less sensitive to the GVL effect, as evidenced by the apparent absence of a protective effect of chronic GVHD on progression in this subgroup. This has also been noted in a series of patients with aggressive NHL19 treated with myeloablative SCT. It must be remembered that many of the patients with aggressive NHL in our study (78%) had failed a prior autograft, which may select for more aggressive disease. Nonetheless, based on our results and those of others, and at least as long as autologous transplantation remains the standard of care for patients in second remission, it may be unlikely that changes in the timing of allogeneic transplantation, in the intensity of conditioning regimen, or in the intensity of post-transplantation immunosuppression (with our current armamentarium) will fundamentally alter the outcome of RIC SCT for this disease, unless new ways to augment the anti-lymphoma immune response can be found.
For patients with mantle cell lymphoma, allogeneic stem cell transplantation has been documented to lead to long-term remissions, with either myeloablative or non-ablative conditioning20. However, reported outcomes vary widely21,22; thus, while it is clear that this entity is sensitive to GVL, it remains unclear whether conditioning regimen intensity is important, and more importantly what the patient characteristics are that would predict for a good outcome after RIC SCT.
It may be surprising that chemosensitivity was not a significant factor for progression or PFS in our series, as has been described previously for both myeloablative and non-ablative SCT2,17. This discrepancy may be partly explained by patient selection, in that patients selected for RIC SCT despite chemorefractoriness may have less aggressive disease progression or smaller tumor bulk, and may hence have a better outcome than unselected refractory patients. Moreover, the methods used for restaging prior to RIC SCT (in particular in the use of PET versus CT scan and the use of bone marrow biopsy) differed among patients in our study; this could have obscured a possible difference in outcome between chemosensitive and chemoreftractory patients.
Our study also calls into question the prognostic relevance of donor chimerism in the setting of RIC SCT for lymphoma. Indeed, even with our low-intensity conditioning, most patients achieved full donor chimerism early. This did not correlate with transplantation outcome. More importantly, there was no apparent loss of chimerism at the time of disease relapse or progression, and no predictive value of chimerism for DLI outcome. Those results suggest that chimerism (at least total leukocyte chimerism) is not an acceptable surrogate for outcome in RIC SCT for lymphoma, and should perhaps not be used to guide treatment decisions other than in the context of graft failure.
Acknowledgments
Support: NHLBI grant HL070149 PA is a recipient of a career development award from the Leukemia and Lymphoma Society
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schmitz N, Dreger P, Glass B, Sureda A. Allogeneic transplantation in lymphoma: current status. Haematologica. 2007;92:1533–1548. doi: 10.3324/haematol.11185. [DOI] [PubMed] [Google Scholar]
- 2.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 3.van Besien K, Loberiza FR, Jr, Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 4.Bierman PJ, Sweetenham JW, Loberiza FR, Jr, et al. Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin's lymphoma: a comparison with allogeneic and autologous transplantation--The Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2003;21:3744–3753. doi: 10.1200/JCO.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 5.Khouri IF, Saliba RM, Lee M-S, et al. Longer Follow-Up Confirms a Low Relapse Rate after Non-Myeloablative Allogeneic Transplantation (NMT) for Non-Hodgkin’s Lymphoma (NHL), Including Patients with PET or Gallium-Avid Disease. Blood (ASH Annual Meeting Abstracts) 2005;106:44. [Google Scholar]
- 6.Brown JR, Kim HT, Li S, et al. Predictors of improved progression-free survival after nonmyeloablative allogeneic stem cell transplantation for advanced chronic lymphocytic leukemia. Biol Blood Marrow Transplant. 2006;12:1056–1064. doi: 10.1016/j.bbmt.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading; Bone Marrow Transplant; 1995. pp. 825–828. [PubMed] [Google Scholar]
- 9.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics. 1988;16:1140–1154. [Google Scholar]
- 10.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 11.Morris E, Thomson K, Craddock C, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004;104:3865–3871. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 12.Maris MB, Sandmaier BM, Storer B, et al. Allogeneic Hematopoietic Cell Transplantation (HCT) after Nonmyeloablative Conditioning for Relapsed or Refractory Follicular Lymphoma. Blood (ASH Annual Meeting Abstracts) 2005;106:1130. [Google Scholar]
- 13.Van Besien K. The evolving role of autologous and allogeneic stem cell transplantation in follicular lymphoma. Blood Rev. 2006;20:235–244. doi: 10.1016/j.blre.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Corradini P, Dodero A, Farina L, et al. Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: pre-transplant disease status and histotype heavily influence outcome. Leukemia. 2007 doi: 10.1038/sj.leu.2404822. [DOI] [PubMed] [Google Scholar]
- 15.Rezvani AR, Storer B, Maris M, et al. Nonmyeloablative Allogeneic Hematopoietic Cell Transplantation in Relapsed, Refractory, and Transformed Indolent Non-Hodgkin's Lymphoma. J Clin Oncol. 2007 doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 16.Baron F, Storb R, Storer B, et al. Allogeneic Hematopoietic Cell Transplantation (HCT) with Nonmyeloablative Conditioning after Failed Myeloablative HCT: Factors Affecting Outcomes. Blood (ASH Annual Meeting Abstracts) 2005;106:1143. [Google Scholar]
- 17.Robinson SP, Goldstone AH, Mackinnon S, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 18.Faulkner RD, Craddock C, Byrne JL, et al. BEAM-alemtuzumab reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases: GVHD, toxicity, and survival in 65 patients. Blood. 2004;103:428–434. doi: 10.1182/blood-2003-05-1406. [DOI] [PubMed] [Google Scholar]
- 19.Doocey RT, Toze CL, Connors JM, et al. Allogeneic haematopoietic stem-cell transplantation for relapsed and refractory aggressive histology non-Hodgkin lymphoma. Br J Haematol. 2005;131:223–230. doi: 10.1111/j.1365-2141.2005.05755.x. [DOI] [PubMed] [Google Scholar]
- 20.Kiss TL, Mollee P, Lazarus HM, Lipton JH. Stem cell transplantation for mantle cell lymphoma: if, when and how? Bone Marrow Transplant. 2005;36:655–661. doi: 10.1038/sj.bmt.1705080. [DOI] [PubMed] [Google Scholar]
- 21.Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 22.Robinson SP, Schmitz N, Taghipour G, Sureda A. Reduced Intensity Allogeneic Stem Cell Transplantation for Mantle Cell Lymphoma Is Associated with Substantial Late Transplant Related Mortality and a Poor Outcome in Patients with Chemoresistant Disease. Blood (ASH Annual Meeting Abstracts) 2004;104:2260. [Google Scholar]
- 23.Anderlini P, Saliba R, Acholonu S, et al. Reduced-intensity allogeneic stem cell transplantation in relapsed and refractory Hodgkin's disease: low transplant-related mortality and impact of intensity of conditioning regimen. Bone Marrow Transplant. 2005;35:943–951. doi: 10.1038/sj.bmt.1704942. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JE, Litzow MR, Appelbaum FR, et al. Allogeneic, syngeneic, and autologous marrow transplantation for Hodgkin's disease: the 21-year Seattle experience. J Clin Oncol. 1993;11:2342–2350. doi: 10.1200/JCO.1993.11.12.2342. [DOI] [PubMed] [Google Scholar]
- 25.Milpied N, Fielding AK, Pearce RM, Ernst P, Goldstone AH. Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed Hodgkin's disease. European Group for Blood and Bone Marrow Transplantation. J Clin Oncol. 1996;14:1291–1296. doi: 10.1200/JCO.1996.14.4.1291. [DOI] [PubMed] [Google Scholar]
- 26.Akpek G, Ambinder RF, Piantadosi S, et al. Long-term results of blood and marrow transplantation for Hodgkin's lymphoma. J Clin Oncol. 2001;19:4314–4321. doi: 10.1200/JCO.2001.19.23.4314. [DOI] [PubMed] [Google Scholar]