Abstract
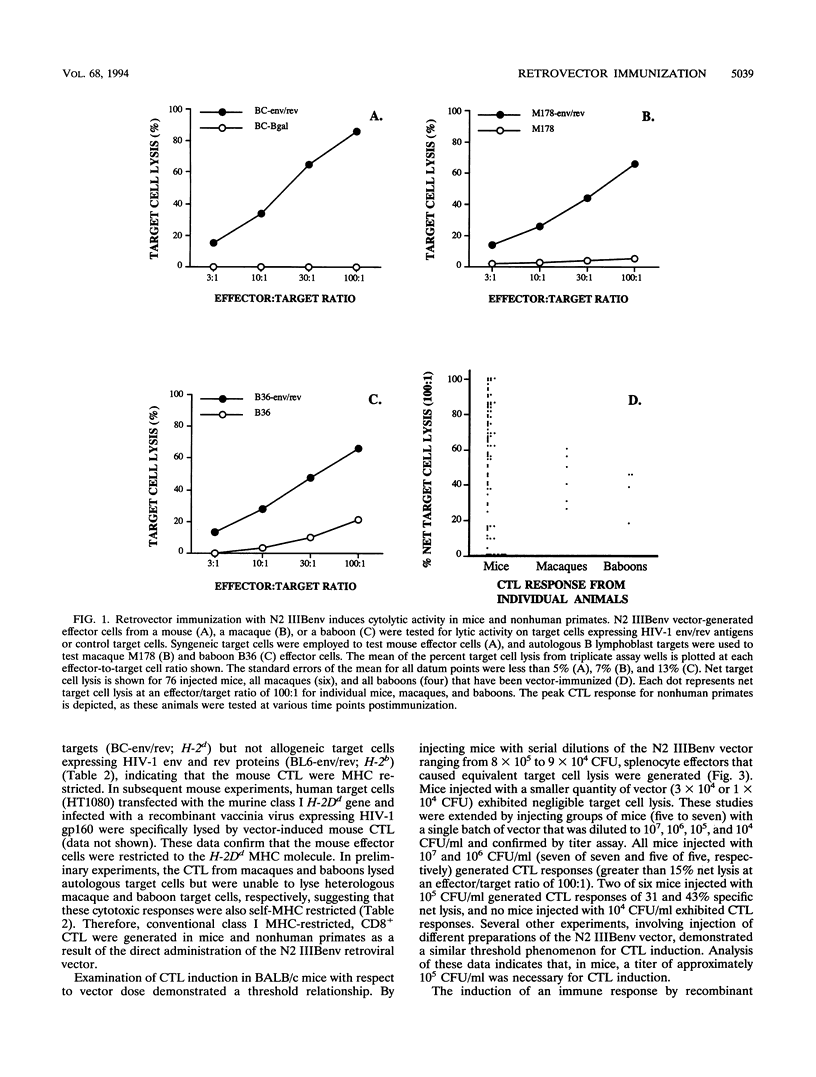
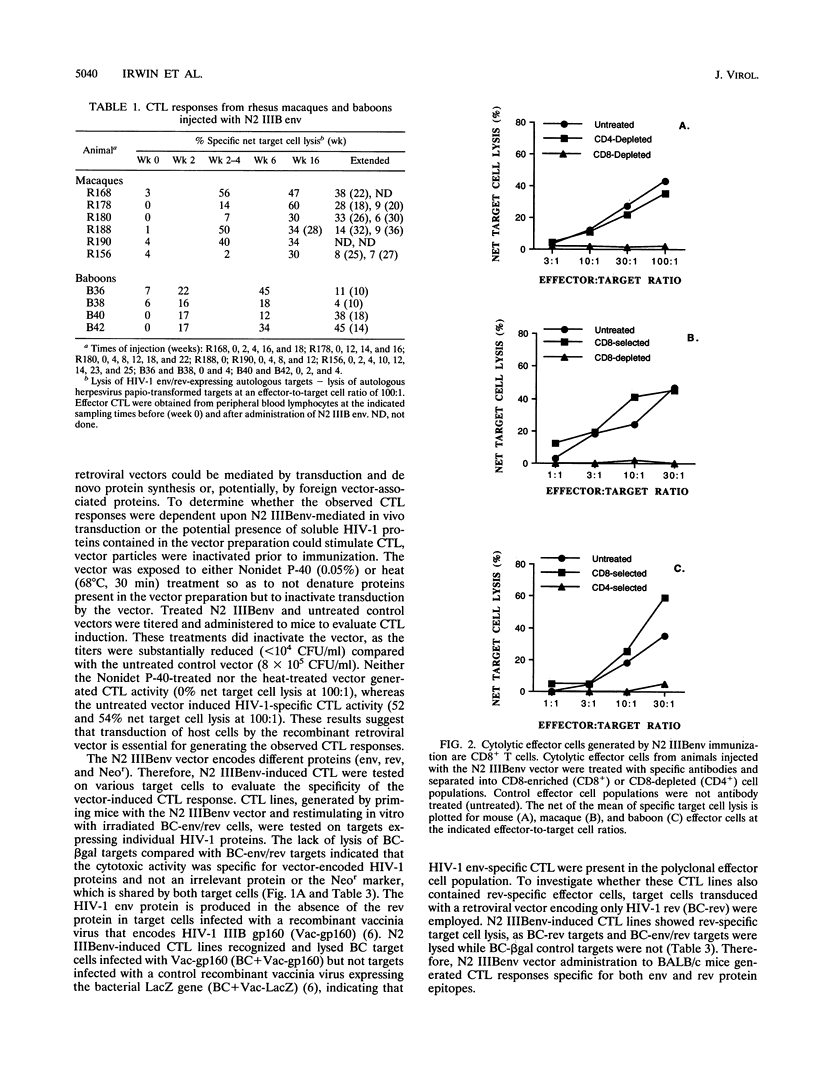
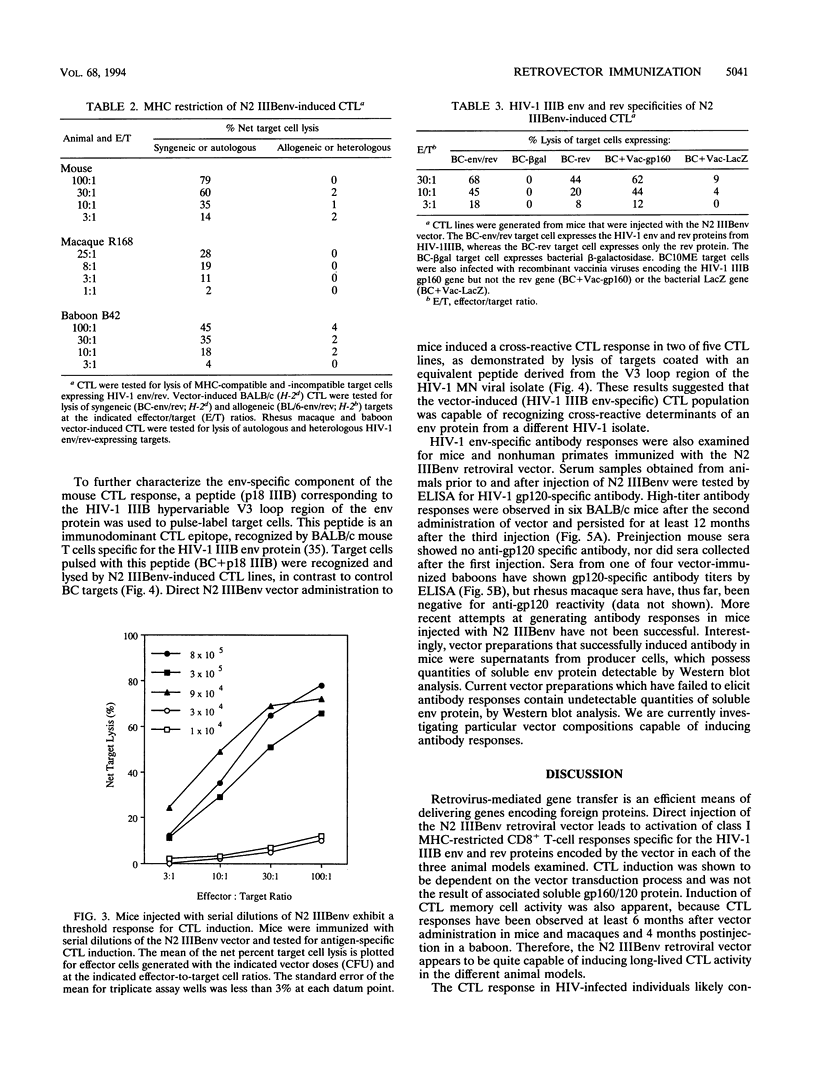
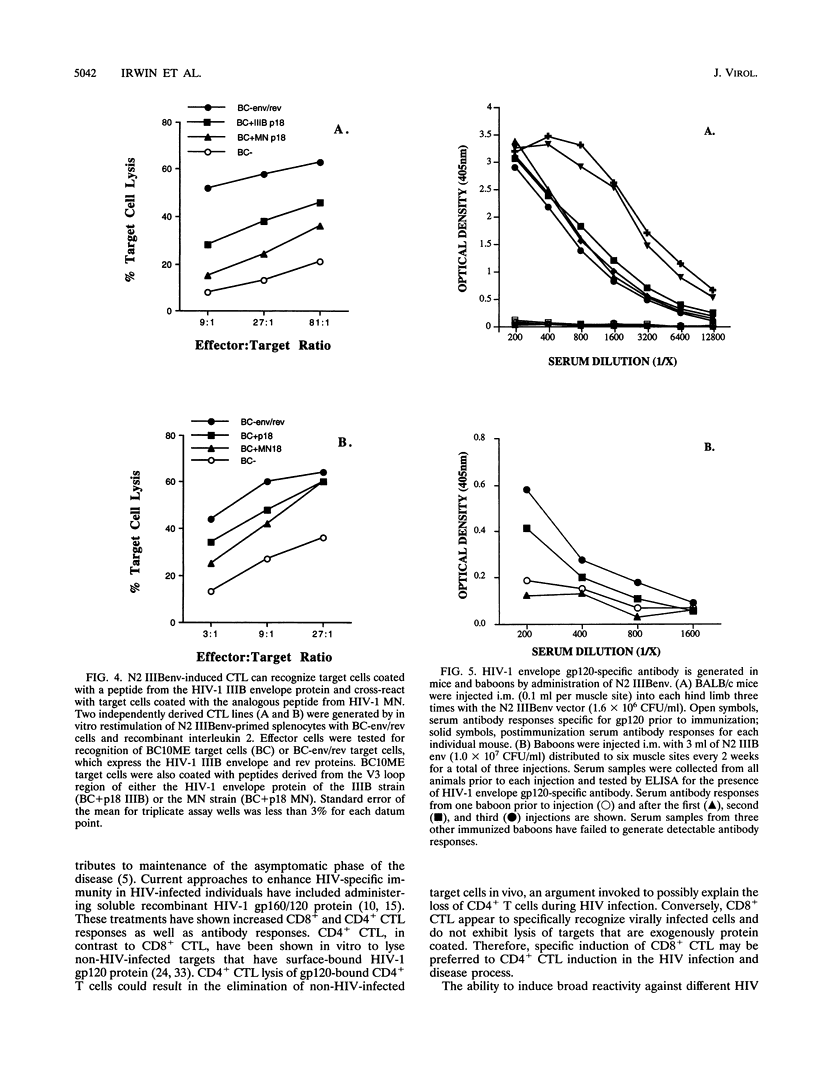
The cytotoxic T-lymphocyte (CTL) response plays an important role in controlling the severity and duration of viral infections. Immunization by direct in vivo administration of retroviral vector particles represents an efficient means of introducing and expressing genes and, subsequently, the proteins they encode in vivo in mammalian cells. In this manner foreign proteins can be provided to the endogenous, class I major histocompatibility complex antigen presentation pathway leading to CTL activation. A nonreplicating recombinant retroviral vector, encoding the human immunodeficiency virus type 1 (HIV-1) IIIB envelope and rev proteins, has been developed and examined for stimulation of immune responses in mouse, rhesus macaque, and baboon models. Animals were immunized by direct intramuscular injection of the retroviral vector particles. Vector-immunized mice, macaques, and baboons generated long-lived CD8+, major histocompatibility complex-restricted CTL responses that were HIV-1 protein specific. The CTL responses were found to be dependent on the ability of the retroviral vector to transduce cells. The vector also elicited HIV-1 envelope-specific antibody responses in mice and baboons. These studies demonstrate the ability of a retroviral vector encoding HIV-1 proteins to stimulate cellular and humoral immune responses and suggest that retrovector immunization may provide an effective means of inducing or augmenting CTL responses in HIV-1-infected individuals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur L. O., Bess J. W., Jr, Waters D. J., Pyle S. W., Kelliher J. C., Nara P. L., Krohn K., Robey W. G., Langlois A. J., Gallo R. C. Challenge of chimpanzees (Pan troglodytes) immunized with human immunodeficiency virus envelope glycoprotein gp120. J Virol. 1989 Dec;63(12):5046–5053. doi: 10.1128/jvi.63.12.5046-5053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman P. W., Groopman J. E., Gregory T., Clapham P. R., Weiss R. A., Ferriani R., Riddle L., Shimasaki C., Lucas C., Lasky L. A. Human immunodeficiency virus type 1 challenge of chimpanzees immunized with recombinant envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5200–5204. doi: 10.1073/pnas.85.14.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Burns J. C., Friedmann T., Driever W., Burrascano M., Yee J. K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael A., Jin X., Sissons P., Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993 Feb 1;177(2):249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada S., DeJesus C. E., Townsend K., Lee W. T., Laube L., Jolly D. J., Chang S. M., Warner J. F. Cross-reactive lysis of human targets infected with prototypic and clinical human immunodeficiency virus type 1 (HIV-1) strains by murine anti-HIV-1 IIIB env-specific cytotoxic T lymphocytes. J Virol. 1993 Jun;67(6):3409–3417. doi: 10.1128/jvi.67.6.3409-3417.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmann B., Gomard E., Kiény M. P., Guy B., Dreyfus F., Saimot A. G., Sereni D., Lévy J. P. An antigenic peptide of the HIV-1 NEF protein recognized by cytotoxic T lymphocytes of seropositive individuals in association with different HLA-B molecules. Eur J Immunol. 1989 Dec;19(12):2383–2386. doi: 10.1002/eji.1830191231. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Hammond S. A., Bollinger R. C., Stanhope P. E., Quinn T. C., Schwartz D., Clements M. L., Siliciano R. F. Comparative clonal analysis of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ and CD8+ cytolytic T lymphocytes isolated from seronegative humans immunized with candidate HIV-1 vaccines. J Exp Med. 1992 Dec 1;176(6):1531–1542. doi: 10.1084/jem.176.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Doherty P. C., Zijlstra M., Jaenisch R., Katz J. M. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992 Aug 15;149(4):1319–1325. [PubMed] [Google Scholar]
- Kast W. M., Bronkhorst A. M., de Waal L. P., Melief C. J. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J Exp Med. 1986 Sep 1;164(3):723–738. doi: 10.1084/jem.164.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavinskis L. S., Whitton J. L., Oldstone M. B. Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection. J Virol. 1989 Oct;63(10):4311–4316. doi: 10.1128/jvi.63.10.4311-4316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S., Fuerst T. R., Wood L. V., Woods R. M., Suzich J. A., Jones G. M., de la Cruz V. F., Davey R. T., Jr, Venkatesan S., Moss B. Mapping the fine specificity of a cytolytic T cell response to HIV-1 nef protein. J Immunol. 1990 Jul 1;145(1):127–135. [PubMed] [Google Scholar]
- Kundu S. K., Katzenstein D., Moses L. E., Merigan T. C. Enhancement of human immunodeficiency virus (HIV)-specific CD4+ and CD8+ cytotoxic T-lymphocyte activities in HIV-infected asymptomatic patients given recombinant gp160 vaccine. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11204–11208. doi: 10.1073/pnas.89.23.11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S. K., Merigan T. C. Inverse relationship of CD8+CD11+ suppressor T cells with human immunodeficiency virus (HIV)-specific cellular cytotoxicity and natural killer cell activity in HIV infection. Immunology. 1991 Dec;74(4):567–571. [PMC free article] [PubMed] [Google Scholar]
- Laube L. S., Burrascano M., Dejesus C. E., Howard B. D., Johnson M. A., Lee W. T., Lynn A. E., Peters G., Ronlov G. S., Townsend K. S. Cytotoxic T lymphocyte and antibody responses generated in rhesus monkeys immunized with retroviral vector-transduced fibroblasts expressing human immunodeficiency virus type-1 IIIB ENV/REV proteins. Hum Gene Ther. 1994 Jul;5(7):853–862. doi: 10.1089/hum.1994.5.7-853. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Fenrick R., Cullen B. R. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature. 1988 Sep 8;335(6186):181–183. doi: 10.1038/335181a0. [DOI] [PubMed] [Google Scholar]
- McMichael A. J. Role of class I molecules of the major histocompatibility complex in cytotoxic T-cell function in health and disease. Springer Semin Immunopathol. 1992;14(1):1–16. doi: 10.1007/BF00197129. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon D. F., Townsend A. R., Elvin J. G., Rizza C. R., Gallwey J., McMichael A. J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988 Dec 1;336(6198):484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- Orentas R. J., Hildreth J. E., Obah E., Polydefkis M., Smith G. E., Clements M. L., Siliciano R. F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp160 subunit vaccine. Science. 1990 Jun 8;248(4960):1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- Patek P. Q., Collins J. L., Cohn M. Activity and dexamethasone sensitivity of natural cytotoxic cell subpopulations. Cell Immunol. 1982 Sep 1;72(1):113–121. doi: 10.1016/0008-8749(82)90288-x. [DOI] [PubMed] [Google Scholar]
- Peebles P. T. An in vitro focus-induction assay for xenotropic murine leukemia virus, feline leukemia virus C, and the feline--primate viruses RD-114/CCC/M-7. Virology. 1975 Sep;67(1):288–291. doi: 10.1016/0042-6822(75)90427-4. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusser P., Riddell S. R., Meyers J. D., Greenberg P. D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991 Sep 1;78(5):1373–1380. [PubMed] [Google Scholar]
- Rook A. H., Lane H. C., Folks T., McCoy S., Alter H., Fauci A. S. Sera from HTLV-III/LAV antibody-positive individuals mediate antibody-dependent cellular cytotoxicity against HTLV-III/LAV-infected T cells. J Immunol. 1987 Feb 15;138(4):1064–1067. [PubMed] [Google Scholar]
- Rouse B. T., Norley S., Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988 Jan-Feb;10(1):16–33. doi: 10.1093/clinids/10.1.16. [DOI] [PubMed] [Google Scholar]
- Sharp R. M., Durocher C. L., Parmenter J. L. Production of baboon (Papio hamadryas) monoclonal antibodies by herpesvirus papio immortalized baboon lymph node cells. J Immunol Methods. 1990 Feb 9;126(2):287–294. doi: 10.1016/0022-1759(90)90162-o. [DOI] [PubMed] [Google Scholar]
- Siliciano R. F., Lawton T., Knall C., Karr R. W., Berman P., Gregory T., Reinherz E. L. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988 Aug 12;54(4):561–575. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- St Louis D., Verma I. M. An alternative approach to somatic cell gene therapy. Proc Natl Acad Sci U S A. 1988 May;85(9):3150–3154. doi: 10.1073/pnas.85.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Cohen J., Hosmalin A., Cease K. B., Houghten R., Cornette J. L., DeLisi C., Moss B., Germain R. N., Berzofsky J. A. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Merli S., Putney S. D., Houghten R., Moss B., Germain R. N., Berzofsky J. A. A single amino acid interchange yields reciprocal CTL specificities for HIV-1 gp160. Science. 1989 Oct 6;246(4926):118–121. doi: 10.1126/science.2789433. [DOI] [PubMed] [Google Scholar]
- Tsubota H., Lord C. I., Watkins D. I., Morimoto C., Letvin N. L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989 Apr 1;169(4):1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Birch-Limberger K., Fisher L., Paradis T. J., Aldovini A., Young R., Moss B., Schooley R. T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Paradis T. J., Fuller T. C., Hirsch M. S., Schooley R. T., Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988 Apr 1;240(4848):64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- Warner J. F., Anderson C. G., Laube L., Jolly D. J., Townsend K., Chada S., St Louis D. Induction of HIV-specific CTL and antibody responses in mice using retroviral vector-transduced cells. AIDS Res Hum Retroviruses. 1991 Aug;7(8):645–655. doi: 10.1089/aid.1991.7.645. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Eichberg J. W., Moran P. A., McClure J., Sridhar P., Hu S. L. Proliferative and cytotoxic T cells to AIDS virus glycoproteins in chimpanzees immunized with a recombinant vaccinia virus expressing AIDS virus envelope glycoproteins. J Immunol. 1987 Aug 15;139(4):988–990. [PubMed] [Google Scholar]



