Abstract
The purpose of this study was to test the hypothesis that elevation in protein oxidative damage during the aging process is a targeted rather than a stochastic phenomenon. Oxidative damage to proteins in mitochondrial membranes in the flight muscles of the housefly, manifested as carbonyl modifications, was detected immunochemically with anti-dinitrophenyl antibodies. Adenine nucleotide translocase (ANT) was found to be the only protein in the mitochondrial membranes exhibiting a detectable age-associated increase in carbonyls. The age-related elevation in ANT carbonyl content was correlated with a corresponding loss in its functional activity. Senescent flies that had lost the ability to fly exhibited a relatively higher degree of ANT oxidation and a greater loss of functional activity than their cohorts of the same age that were still able to fly. Exposure of flies to 100% oxygen resulted in an increase in the level of ANT carbonyl content and a loss in its activity. In vitro treatment of mitochondria with a system that generated hydroxyl free radicals caused an increase in ANT carbonyl level and a decrease in ANT exchange activity. ANT was also the only mitochondrial membrane protein exhibiting adducts of the lipid peroxidation product 4-hydroxynonenal. Results of this study indicate that proteins in mitochondrial membranes are modified selectively during aging.
Presently, the nature of the mechanisms causing losses in the efficiency of various biological functions during the aging process is poorly understood. One hypothesis suggests that accrual of molecular oxidative damage is a major causal factor in the senescence-related decline of physiological fitness of organisms (1–3). It is widely believed that the infliction of molecular oxidative damage, associated with aging, is a random rather than a selective or controlled phenomenon (4). Accordingly, validation of this hypothesis has been sought on the basis of measurements of oxidative damage in tissue homogenates. Indeed, studies in several laboratories have reported age-associated increases in concentrations of oxidation products of macromolecules such as DNA (5), lipids (6), and proteins (7) in the homogenates of different tissues. Many have interpreted these increases as indicating that oxidative damage during aging is a stochastic phenomenon.
Oxidative damage to proteins has been postulated to be of key importance in the aging process, because oxidized proteins often lose enzyme activity and may be targeted for preferential degradation (8, 9). Attacks of reactive oxygen species on proteins have been shown to increase their carbonyl content because of the formation of aldehydes and ketones in certain amino acid residues (8, 9). Age-associated increases in protein carbonyls, reported in several model systems, are thus believed to be of particular physiological relevance (8, 9). Studies on the age-related pattern of activities of various enzymes have indicated no uniform trend (10). Indeed, activities of most of the enzymes remain unaltered. However, some show an increase, and only a few exhibit a decline during aging (11). Such a diversified pattern is, seemingly, inconsistent with the view that protein oxidative damage occurs randomly.
The present study tests the hypothesis that the age-associated oxidative damaging of proteins, detected as carbonyl modifications, is a selectively targeted rather than a randomly directed process. Mitochondrial membrane proteins from the flight muscles of the housefly were chosen as a model to test this hypothesis for a number of reasons: (i) mitochondria have been implicated in degenerative diseases and aging (12); (ii) the inner mitochondrial membrane, being the major intracellular site for the generation of superoxide anion radical (13–15), a progenitor of other reactive oxygen species (16), would be vulnerable to oxidative attacks; and (iii) flight-muscle mitochondria in dipteran flies consume oxygen at one of the highest rates known in biological systems (17) and also produce O2⨪/H2O2 at a high rate (18). The age-associated accrual of oxidative damage could thus be predicted to occur in this model system. Results of the present study indicate that age-related increase in molecular oxidative damage to mitochondrial membrane proteins is indeed highly selective, primarily involving adenine nucleotide translocase (ANT), with severe loss of its functional activity.
MATERIALS AND METHODS
Materials.
[2,8-3H]Adenosine 5′-diphosphate ([3H]ADP) trisodium salt and atractyloside were purchased from Sigma. Rat anti-2,4-dinitrophenyl (DNP) monoclonal antibody (IgG) and mouse anti-rat IgG monoclonal antibody, conjugated with horseradish peroxidase, were obtained from Zymed. Anti-4-hydroxynonenal (anti-HNE) polyclonal antibodies were a kind gift from Luke Szweda. Adult male houseflies (Musca domestica) were used in all experiments.
Immunochemical Detection of Protein Carbonyls.
Mitochondrial membranes, isolated as described (19), were solubilized in 10 mM sodium phosphate buffer, pH 7.4, containing 1% Triton X-100 and 1 mM EDTA, followed by treatment with 10 mM 2,4-dinitrophenylhydrazine (DNPH) according to Levine et al. (20). The DNPH derivatized sample, dissolved in 20 mM Tris⋅HCl buffer, pH 7.4, containing 0.1% SDS, was electrophoresed by SDS/PAGE according to Laemmli (21). Proteins were then transferred to Immobilon-P membranes (Millipore)according to Towbin et al. (22). Immunochemical detection was performed as described (19). Protein-HNE adducts were detected with anti-HNE antibodies (23). An AlphaImager 2000 (Alpha Innotech, San Leandro, CA) was used for all densitometric quantitations.
Purification and Identification of the Protein.
The 33-kDa protein shown in Fig. 1 was purified as follows. Mitochondrial membranes were solubilized in 20 mM 1,3-diaminopropane buffer (pH 10.5, containing 1% Triton X-100 and 1 mM EDTA). The solution was incubated in ice for 60 min and then centrifuged for 60 min at 105,000 × g (24). Another pellet was extracted under the same conditions, and the resulting supernatants were pooled and applied to a diethylaminoethyl-Sepharose fast-flow column (Pharmacia), which was preequilibrated with the diaminopropane buffer. The column was washed with the diaminopropane buffer containing an increasing concentration of NaCl (0–500 mM, step gradient with 20 ml of elution buffer in each step; the increment of NaCl was 100 mM, diluted from a 2 M stock solution). Fractions containing the 33-kDa protein were pooled, changed into 50 mM Hepes buffer (pH 7.5, containing 1% Triton X-100 and 1 mM EDTA) by using PD-10 columns, and applied to a carboxymethyl-Sepharose fast-flow column, which was preequilibrated with the Hepes buffer. The column was eluted by the Hepes-buffer solution containing increasing concentration of NaCl (step gradient, as above).
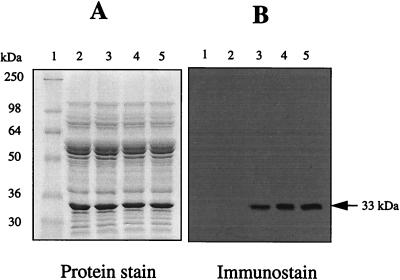
Figure 1.
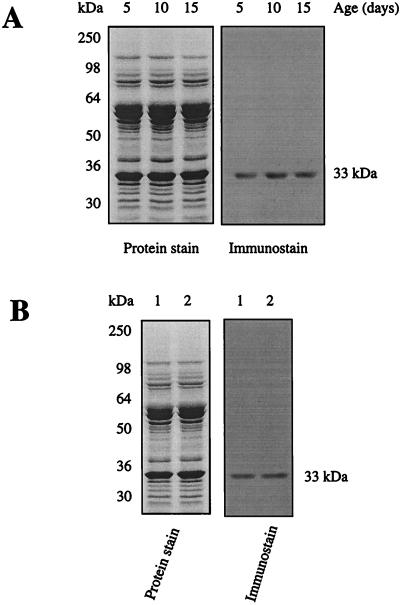
Immunochemical detection of protein carbonyls in mitochondrial membranes from flight muscles of houseflies. DNPH-treated proteins (10 μg) were electrophoresed on SDS/PAGE (10% resolving gel) under reducing conditions and transferred to Immobilon-P membrane. Oxidized proteins were detected immunochemically as described in the text. Lane 1, protein standard markers; lane 2, proteins without DNPH treatment; lanes 3–5, 5-, 10-, and 15-day-old fly mitochondrial proteins, respectively, treated with DNPH. A 33-kDa protein in the membranes exhibited a strong immunoreaction for the carbonyl groups.
Further purification was achieved by gel electrophoresis (25) of the fractions eluted from the carboxymethyl-Sepharose column. Essentially, fractions containing the 33-kDa protein were pooled, desalted by using PD-10 columns, and analyzed by reverse isoelectric focusing according to Madden (26). The 33-kDa protein band in the isoelectric focusing gel (Fig. 2B) was excised, equilibrated for 30 min in a solution containing 5% 2-mercaptoethanol, 62.5 mM Tris⋅HCl, pH 6.8, 2.3% SDS, and 10 mM glycerol, and loaded onto SDS/PAGE (ref. 25; Fig. 2C). The N-terminal amino acid sequence of this protein was determined by automated Edman degradation, which identified the protein as mitochondrial ANT.
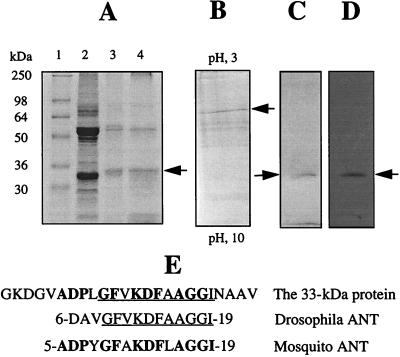
Figure 2.
Purification and identification of the 33-kDa protein as housefly mitochondrial ANT. (A) Lane 1, standard markers; lane 2, crude membrane proteins; lane 3, eluate from DEAE column; lane 4, eluate from carboxymethyl column. (B) Reverse isoelectric focusing analysis of the eluate from carboxymethyl column. Arrow indicates the focused 33-kDa protein. (C) The focused band in B was excised from reverse isoelectric focusing gel and analyzed further by SDS/PAGE. Arrow indicates the purified protein. (D) The purified protein was immunochemically confirmed to be carbonylated. A, B, and C are protein stained by Coomassie blue; D is immunostain. (E) A computer-assisted search from protein database. The underlined amino acids show the N-terminal amino acid sequence homology in mitochondrial ANT from the housefly and Drosophila; the bold amino acids show homology with the mosquito.
Assay of ANT Exchange Activity.
The activity of ANT was measured by a slight modification of the inhibitor-stop method (27). Briefly, mitochondria were suspended in ice-cold reaction buffer (110 mM KCl/20 mM Tris⋅HCl/1 mM EDTA, pH 7.4) and 50 nmol of [3H]ADP was added to a reaction medium consisting of the buffer and 0.5 mg/ml of mitochondrial proteins. The reaction was carried out on ice and stopped after 10 sec by 100 μM atractyloside. The reaction mixture was then centrifuged at 10,000 rpm on a Micro14 benchtop centrifuge (Fisher Scientific) for 5 min, and the supernatant was discarded. Following six washes in ice-cold buffer, the pellet was dissolved in 0.2 ml of 0.1 M NaOH and counted for radioactivity.
Other Methods.
Flies were exposed to 100% oxygen as described (19). In vitro oxidation of mitochondria was carried out with the vanadyl/H2O2 system (28). Membranes were then isolated, and the concentration of carbonyls was measured spectrophotometrically (20). Protein concentrations were determined by bicinchoninic acid assay (29) with BSA as the standard. For statistical analysis, differences between the means were calculated by one-way ANOVA and Student’s unpaired t test. Values of P < 0.05 were considered to be significant.
RESULTS
Identification of ANT as a Target of Oxidative Damage During Aging.
To determine whether various mitochondrial membrane proteins were equally or differentially sensitive to oxidative damage during aging, the carbonyl content of proteins, obtained from 5-, 10-, and 15-day-old flies, was determined with an immunochemical assay. As expected, a considerable number of protein bands were detected by Coomassie-blue staining; however, only a single major protein, which had a molecular mass of ≈33 kDa, was found to be strongly positive for carbonyls, and the increase of its immunostain intensity was found to be age-associated (Fig. 1). No immunostaining was detectable in the control samples, which were not exposed to DNPH (Fig. 1).
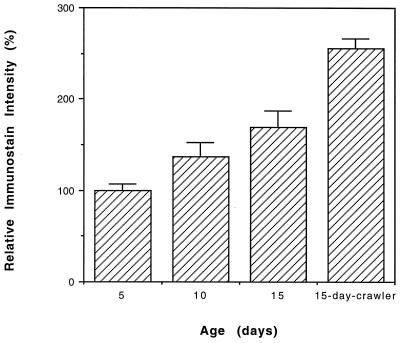
A comparison of the immunostained preparations from 5-, 10-, and 15-day-old flies, representing young, middle-aged, and old animals (30, 31), indicated an increase in relative carbonyl content of the 33-kDa protein with increasing age (Fig. 3). There were 36.5% and 69% carbonyl-content increases in protein obtained from 10-day- and 15-day-old flies, respectively, as compared with that obtained from 5-day-old flies (Fig. 3). A densitometric comparison between 15-day-old flies that had lost the ability to fly (“crawlers”) and their cohorts that still retained the ability to fly (“flyers”) showed that the carbonyl content of this protein was 50% higher in the crawlers than in the flyers (Fig. 3). The purified 33-kDa protein (Fig. 2C), confirmed to be the major species for carbonylation (Fig. 2D), was subjected to N-terminal microsequencing that allowed its identification as mitochondrial ANT (Fig. 2E).
Figure 3.
Immunochemical estimation of the carbonyl content of the 33-kDa protein in housefly at different ages. Presented values are mean ± SD of relative densitometric determinations in four independent experiments that used flies hatched on different dates. Immunostain intensity of the protein from 5-day-old flies was assigned an arbitrary value of 100; the ratio of 10- or 15-day-old flies and crawlers to the 5-day-old flies is reported. The increase in ANT carbonyl content between each age group was statistically significant.
Effect of Chronological and Physiological Age of Flies on ANT Activity.
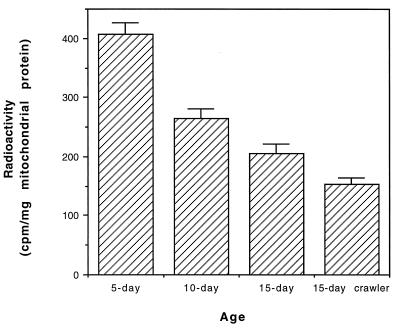
To determine whether the observed age-related increase in the carbonyl content of ANT was associated with a corresponding loss of ANT activity, the ADP/ATP exchange activity of ANT was measured with the inhibitor-stop method (27). A comparison of ANT activity in mitochondria obtained from 5-, 10-, and 15-day-old flies indicated a gradual loss of activity, whereby the 15-day-old flies exhibited only 48% of the activity of the 5-day-old flies (Fig. 4). Moreover, ANT exchange activity was 26% lower in the 15-day-old crawlers than in 15-day-old flyers (Fig. 4). Thus, the age-related decrease in ANT functional activity was related to both the chronological and the physiological functional age of the flies as well as to ANT carbonylation.
Figure 4.
Exchange activity of mitochondrial ANT in the flight muscles of the houseflies at different ages. See text for the detailed method. Values are the mean ± SD of four experiments that used flies hatched on different dates. The drop in exchange activity between each age group was statistically significant.
Hyperoxic Treatment and ANT Inactivation.
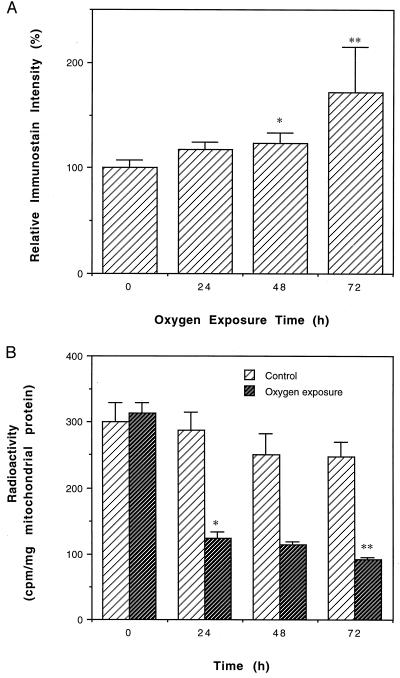
Hyperoxia has been shown to elevate the rate of mitochondrial O2⨪ and H2O2 generation as well as the amount of mitochondrial carbonyl content (31). Exposure of adult houseflies to 100% oxygen has been reported to result in a total mortality within 4–5 days (30). It was reasoned that if inactivation of ANT during normal aging is caused by oxidative damage, exposure of houseflies to hyperoxia would be expected to elevate ANT carbonyl content and to enhance ANT inactivation. This hypothesis was tested by exposing flies to 100% ambient oxygen for varying periods. Compared with the controls, there were, respectively, 18%, 23%, and 72% increases in ANT carbonylation in flies exposed to 100% oxygen for 24, 48, and 72 h (Fig. 5A). During the same period, ADP/ATP exchange activity was greatly decreased, with only 27% activity remaining after 72 h of exposure to 100% oxygen (Fig. 5B).
Figure 5.
Effects of hyperoxia on ANT carbonyl formation and ANT activity. (A) Immunochemical estimation of ANT carbonyls in houseflies exposed to 100% ambient oxygen. Shown are relative densitometric values expressed as mean ± SD of four independent experiments. Immunostain intensity of the control was assigned an arbitrary value of 100, the ratio of oxygen-treated to control is reported. Flies that were 9 days old were exposed to 100% oxygen for indicated periods. (∗, P < 0.05 vs. 0 h; ∗∗, P < 0.01 vs. 48 h.) The changes between 0 and 24 h and between 24 and 48 h were not statistically significant. (B) Effect of 100% oxygen on the exchange activity of mitochondrial ANT. Shown are the mean ± SD of four independent determinations. (∗, P < 0.001 vs. control; ∗∗, P < 0.005 vs. 48 h.) The changes between each group of the control (no oxygen exposure) and between 24 and 48 h of oxygen exposure were not statistically significant.
Metal-Catalyzed Oxidation of ANT in Vitro.
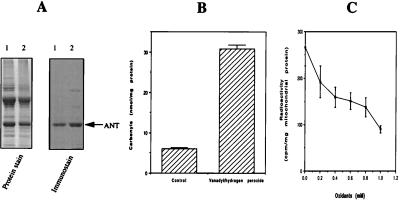
To determine whether the oxidation of housefly ANT and its activity loss in vivo might be mediated by metal-catalyzed oxidation, whole mitochondria were incubated with a system containing vanadyl sulfate and H2O2 that generated hydroxyl free radicals (28). Incubation with this system was followed by either immunochemical detection or ANT activity measurements. For immunochemical detection, mitochondrial membranes were isolated after the in vitro oxidation. ANT was found to be extensively oxidized by the vanadyl/H2O2 system (Fig. 6A). In agreement with previous findings (32), exposure to the system that generated hydroxyl free radicals also caused an attenuation of the staining of oxidized proteins by Coomassie blue (Fig. 6A Left). Nevertheless, there was a large increase in the protein carbonyl content in the oxidized membranes (Fig. 6B), suggesting that ANT carbonylation in vivo could be caused by attacks from hydroxyl free radicals. This in vitro system, as expected, also caused minor oxidations of two other mitochondrial membrane proteins (Fig. 6A, Right). Fig. 6C shows that there was a great drop in ANT activity after incubation of whole mitochondria with this in vitro oxidation system.
Figure 6.
Metal-catalyzed oxidation of mitochondrial membrane proteins and inactivation of ANT. Mitochondria were incubated with 1 mM vanadyl sulfate and 1 mM H2O2, followed by either membrane isolation or ANT activity measurements. (A) Immunochemical detection of ANT without oxidation (lane 1) and with oxidation (lane 2). (B) Increase of total mitochondrial membrane protein carbonyls after oxidation. Carbonyls were determined spectrophotometrically. (C) Loss of ANT activity caused by metal-catalyzed oxidation. Whole mitochondria were incubated with various concentrations of the oxidants (vanadyl/H2O2 = 1:1) before ANT activity measurements. For both B and C, values are the mean ± SD of four independent determinations that used 10-day-old flies hatched on different dates.
In Vivo Modification of ANT by HNE.
Because ANT is a membrane protein and because lipid peroxidation of mitochondrial membranes has been reported to increase with age (33), the possibility that the carbonyl groups of ANT might have originated partially from lipid peroxidation products, such as HNE, was examined by using anti-HNE antibodies that recognize only the HNE adducts (34). Results indicated that ANT was the only mitochondrial-membrane protein exhibiting detectable protein-bound HNE (Fig. 7). Densitometric quantitation of HNE immunostain showed that there were no notable differences in HNE adducts of ANT in flies of different ages or between flyers and crawlers.
Figure 7.
Immunochemical detection of HNE adducts in ANT. HNE detection was performed as described (23). Rabbit anti-HNE antibody (IgG) was diluted to 1:10,000 in Tris-buffered saline containing 0.1% Tween-20 with overnight exposure of the blot at 4°C. Goat anti-rabbit IgG conjugated with horseradish peroxidase was diluted 1:50,000 in Tris-buffered saline containing 0.1% Tween-20 with 3 h incubation at room temperature. (A) Age-associated changes in HNE level in ANT. (B) Comparison of HNE adducts in ANT between 15-day-old flies (lane 1) and crawlers (lane 2).
DISCUSSION
The results of this study indicate that among the various proteins in the mitochondrial membranes, only ANT could be discerned to exhibit an age-related increase in carbonyl content. Hypothetically, it is possible that the threshold sensitivity of immunostaining employed here may have precluded the detection of some proteins with a relatively minor degree of oxidative damage. However, the relative amount of a specific protein does not seem to be a criterion in the detection of oxidative damage. For instance, another comparatively abundant protein in the mitochondrial membranes, observed by Coomassie-blue staining and with a molecular mass of ≈55 kDa (Fig. 1A), did not exhibit any detectable protein carbonylation. Similarly, in a previous study on the matrix fraction of these mitochondria, aconitase was found to be the only protein showing an age-associated increase in oxidation (19). Thus, age-related accrual of oxidative damage to various mitochondrial proteins in the housefly is differential and affects only a few targets. This finding supports the hypothesis that accrual of protein oxidative damage during aging is highly selective. The hypothetical possibility that some protein(s) of very low abundance but with high degree of carbonylation may have remained undetected does not contradict the inference drawn here—namely, that oxidative damage to protein is selective.
Carbonylation of ANT under hyperoxia increases significantly between 48 and 72 h. In contrast, the ANT activity declines most sharply during the first 24 h. This apparent incongruity may be caused by the fact that the initial damage occurs near the active site of the protein, where it has a much greater impact on the function than does damage elsewhere. This issue may be resolved by identifying the specific amino acid residues of ANT showing carbonyl modification. The relatively small increase in carbonyl content of ANT during the initial phase of hyperoxia may be caused by the fact that ANT, being a transmembrane protein, has an extensive hydrophobic domain that is protected by the antioxidants present in the membrane, such as coenzyme Q and α-tocopherol (35). During the early phase of hyperoxia, this antioxidative protection may remain effective. However, prolonged hyperoxia would enhance lipid peroxidation, a chain reaction, and eventually overcome and/or deplete antioxidative defenses. Indeed, data seem to fit this hypothetical scenario.
An obvious question that arises from the present finding is why ANT selectively undergoes a progressive carbonyl augmentation with age and is relatively more susceptible to oxidative damage. One factor may be the chemical structure and topography of ANT. Studies on the mechanism of protein carbonyl formation have revealed that it involves only certain proteins and occurs by site-specific metal-catalyzed oxidations of arginine, proline, and lysine residues (36, 37). Such a mechanism may also be operative in the housefly, because ANT contains a relatively large number of these three amino acid residues (data not shown). Furthermore, the finding that in vitro exposure of mitochondria to a system that generated hydroxyl free radicals (vanadyl/H2O2) caused a notable increase in ANT carbonyl formation is also consistent with the predictions of this model. Rat-heart ANT has been shown to be modified extensively by an oxidation system containing Cu2+ and tert-butyl hydroperoxide (38); this study suggested that the reaction between membrane protein and lipid peroxidation products is the main cause of ANT modification. ANT is known to be tightly bound to six molecules of cardiolipin, which contains highly unsaturated fatty acids (39). Another possible mechanism by which carbonyl groups may be added to proteins involves covalent interactions between lipid peroxidation products (e.g., HNE and certain amino acid residues; refs. 40–42). The finding that no age-related increase of HNE adducts in ANT was observed suggests that some additional mechanism(s) is responsible for the observed increase of ANT carbonylation. Indeed, according to Stadtman, HNE usually contributes no higher than 5–10% of the protein carbonyl content (43). Nevertheless, the detection of HNE adducts solely in ANT shows that HNE modification of mitochondrial-membrane proteins in vivo is also a selective process, which agrees with the phenomenon observed in this study—namely that whatever the source of the carbonylation may be, the age-associated increase in carbonylation occurs only in specific proteins.
A relevant issue arising from this study is why oxidative damage to specific proteins increases during aging. Results of previous studies suggest that such an elevation in oxidative damage may be determined by an interplay among several different factors. One cause may be the discrepancy between relative rates of oxidation and proteolysis of the specific protein. Mild protein oxidation has been shown to be a marking step in the preferential degradation of oxidized proteins, whereas severe oxidative damage may lead to cross-linking and resistance to proteolysis (8, 9). Loss of neutral/alkaline protease activity has been reported to occur in some mammalian tissues (44). However, in the housefly, no such loss was detected with oxidized BSA as a substrate (44), which implies that age-related decrease in proteolysis may not be a universal phenomenon. Another factor may be the location of the protein and the ambient redox state. ANT is an integral protein in the inner mitochondrial membrane, which is the primary intracellular site for the generation of O2⨪ (14, 45), the progenitor of a cascade of other reactive oxygen species. The rate of mitochondrial O2⨪/H2O2 generation has been shown to increase ≈75% during aging in the housefly (46). This increase correlates with a corresponding increase in oxidative damage to mitochondrial DNA (47) as well as to specific proteins such as ANT and aconitase. The present finding that exposure of houseflies to 100% oxygen caused an increase in the amount of ANT carbonyl content and a loss of activity supports the existence of a relationship between O2⨪/H2O2 generation and protein oxidation. An additional contributory factor may be the decline in antioxidative defenses with the increase in protein oxidative damage. Compared with the young, tissues from old houseflies and mammals have been shown to sustain relatively greater damage in response to exogenous oxidative stress, induced by exposure to x-rays (48, 49), suggesting a decline in overall antioxidative capacity. Taken together, it seems that an increased imbalance between prooxidant generation and antioxidant defenses is the most likely cause for the increase in the steady-state amounts of oxidative damage during aging.
This study identifies mitochondrial ANT as a target of oxidative damage during aging and shows that ANT oxidation and functional inactivation are associated with both the chronological age as well as the physiological age of houseflies. The decrease in ANT activity is likely to have global deleterious functional consequences, because an efficient supply of ADP is necessary to maintain an optimal rate of mitochondrial state-3 respiration as well as the adequate release of ATP to the cytosol. Identification of such modified proteins could facilitate understanding of the mechanisms by which oxidative molecular damage results in loss of cellular functions during aging and may also provide potential biomarkers to assess the rate of the aging process.
Acknowledgments
We thank Karen West and Jeffrey Hulmes for their help with peptide microsequencing and amino acid analysis. We thank Dr. Luke Szweda for providing the anti-HNE antibodies. This work was supported by National Institute on Aging Grant R01AG13563.
ABBREVIATIONS
- ANT
adenine nucleotide translocase
- DNP
2,4-dinitrophenyl
- DNPH
2,4-dinitrophenylhydrazine
- HNE
4-hydroxynonenal
References
- 1. Harman D. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Sohal R S, Weindruch R. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckman K B, Ames B N. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 4.Harman D. Drugs Aging. 1993;3:60–80. doi: 10.2165/00002512-199303010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Beckman K B, Ames B N. J Biol Chem. 1997;272:19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 6.Sheldahl J A, Tappel A L. Exp Gerontol. 1974;9:33–41. doi: 10.1016/0531-5565(74)90005-9. [DOI] [PubMed] [Google Scholar]
- 7.Stadtman E R. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 8.Berlett B S, Stadtman E R. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 9.Stadtman E R, Berlett B S. Chem Res Toxicol. 1997;10:485–494. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- 10.Calleja M, Pena P, Ugalde C, Ferreiro C, Marco R, Garesse R. J Biol Chem. 1993;268:18891–18897. [PubMed] [Google Scholar]
- 11.Gafni A. Rev Biol Res Aging. 1990;4:315–336. [Google Scholar]
- 12.Wallace D C, Brown M D, Melov S, Graham B, Lott M. Biofactors. 1998;7:187–190. doi: 10.1002/biof.5520070303. [DOI] [PubMed] [Google Scholar]
- 13.Wallace D C, Melov S. Nat Genet. 1998;19:105–106. doi: 10.1038/448. [DOI] [PubMed] [Google Scholar]
- 14.Turrens J F, Boveris A. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chance B, Sies H, Boveris A. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 16.Fridovich I. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 17.Davis R A, Fraenkel G. J Exp Biol. 1940;17:402–407. [Google Scholar]
- 18.Sohal R S, Brunk U T. Mutat Res. 1992;275:295–304. doi: 10.1016/0921-8734(92)90033-l. [DOI] [PubMed] [Google Scholar]
- 19.Yan L-J, Levine R L, Sohal R S. Proc Natl Acad Sci USA. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine R L, Garland D, Oliver C N, Amici A, Climent I, Lenz A-G, Ahn B-W, Shaltiel S, Stadtman E R. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida K, Szweda L I, Chae H-Z, Stadtman E R. Proc Natl Acad Sci USA. 1993;90:8742–8746. doi: 10.1073/pnas.90.18.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hjelmeland L M. Methods Enzymol. 1990;182:253–264. doi: 10.1016/0076-6879(90)82021-s. [DOI] [PubMed] [Google Scholar]
- 25.Yan L J, Orr W C, Sohal R S. Anal Biochem. 1998;263:67–71. doi: 10.1006/abio.1998.2799. [DOI] [PubMed] [Google Scholar]
- 26.Madden M S. Anal Biochem. 1995;229:203–206. doi: 10.1006/abio.1995.1403. [DOI] [PubMed] [Google Scholar]
- 27.Duan J, Karmazyn M. Can J Physiol Pharmacol. 1989;67:704–709. doi: 10.1139/y89-114. [DOI] [PubMed] [Google Scholar]
- 28.Winn L M, Wells P G. Free Radical Biol Med. 1997;22:607–621. doi: 10.1016/s0891-5849(96)00340-1. [DOI] [PubMed] [Google Scholar]
- 29.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 30.Sohal R S, Agarwal S, Dubey A, Orr W C. Proc Natl Acad Sci USA. 1993;90:7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohal R S, Dubey A. Free Radical Biol Med. 1994;16:621–626. doi: 10.1016/0891-5849(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 32.Davies K J, Lin S W, Pacifici R E. J Biol Chem. 1987;262:9914–9920. [PubMed] [Google Scholar]
- 33.Bindoli A. Free Radical Biol Med. 1988;5:247–261. doi: 10.1016/0891-5849(88)90018-4. [DOI] [PubMed] [Google Scholar]
- 34.Uchida K, Itakura K, Kawakishi S, Hiai H, Toyokuni S, Stadtman E R. Arch Biochem Biophys. 1995;324:241–248. doi: 10.1006/abbi.1995.0036. [DOI] [PubMed] [Google Scholar]
- 35.Lass A, Sohal R S. Arch Biochem Biophys. 1998;352:229–236. doi: 10.1006/abbi.1997.0606. [DOI] [PubMed] [Google Scholar]
- 36.Stadtman E R. Free Radical Biol Med. 1990;9:315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- 37.Stadtman E R. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 38.Giron-Calle J, Zwizinski C W, Schmid H H O. Arch Biochem Biophys. 1994;315:1–7. doi: 10.1006/abbi.1994.1463. [DOI] [PubMed] [Google Scholar]
- 39.Beyer K, Klingenberg M. Biochemistry. 1985;24:3821–3826. doi: 10.1021/bi00336a001. [DOI] [PubMed] [Google Scholar]
- 40.Szweda L I, Uchida K, Tsai L, Stadtman E R. J Biol Chem. 1993;268:3342–3347. [PubMed] [Google Scholar]
- 41.Uchida K, Stadtman E R. Proc Natl Acad Sci USA. 1992;89:5611–5615. doi: 10.1073/pnas.89.12.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchida K, Stadtman E R. Proc Natl Acad Sci USA. 1992;89:4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stadtman E R. In: Molecular Aspects of Aging: The Status of Oxidatively Modified Proteins as a Marker of Aging. Esser K, Martin G M, editors. New York: Wiley; 1995. pp. 129–142. [Google Scholar]
- 44.Agarwal S, Sohal R S. Arch Biochem Biophys. 1994;309:24–28. doi: 10.1006/abbi.1994.1078. [DOI] [PubMed] [Google Scholar]
- 45.Turrens J F, Freeman B A, Levitt J G, Crapo J D. Arch Biochem Biophys. 1982;217:401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- 46.Sohal R S, Sohal B H. Mech Ageing Dev. 1991;57:187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal S, Sohal R S. Proc Natl Acad Sci USA. 1994;91:12332–12335. doi: 10.1073/pnas.91.25.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal S, Sohal R S. Biochem Biophys Res Commun. 1993;194:1203–1206. doi: 10.1006/bbrc.1993.1950. [DOI] [PubMed] [Google Scholar]
- 49.Agarwal S, Sohal R S. Exp Gerontol. 1996;31:387–392. doi: 10.1016/0531-5565(95)02039-x. [DOI] [PubMed] [Google Scholar]