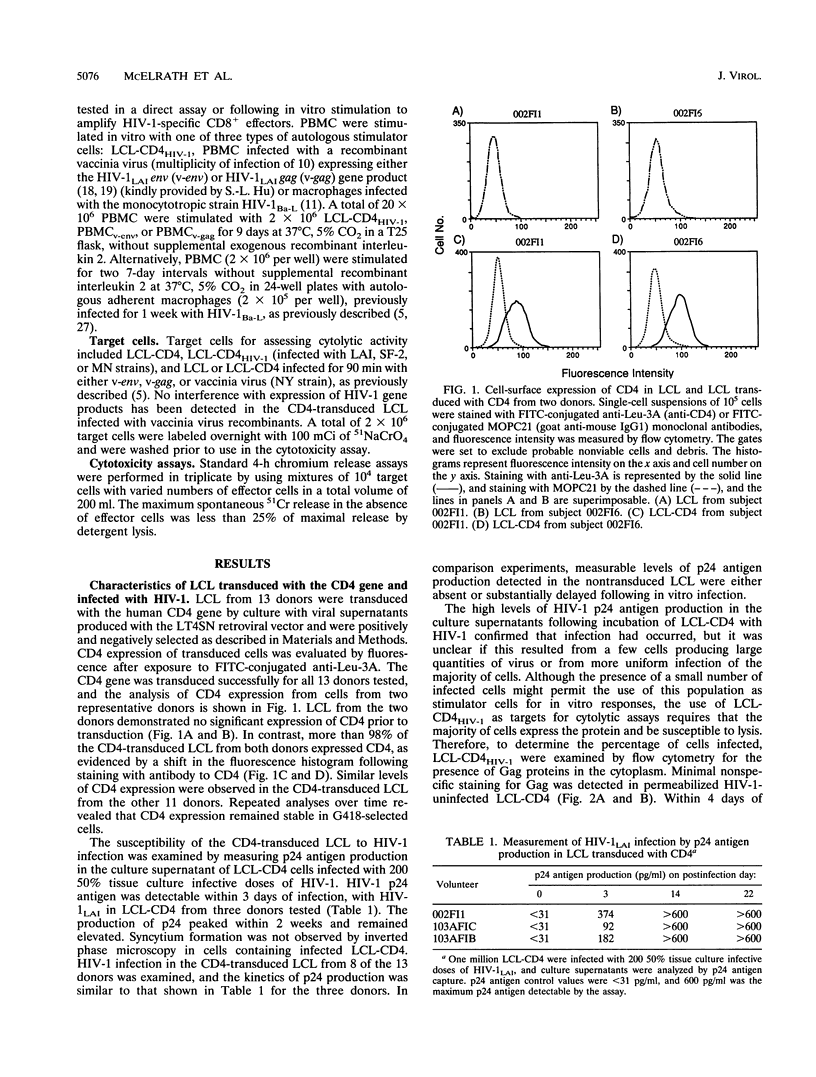
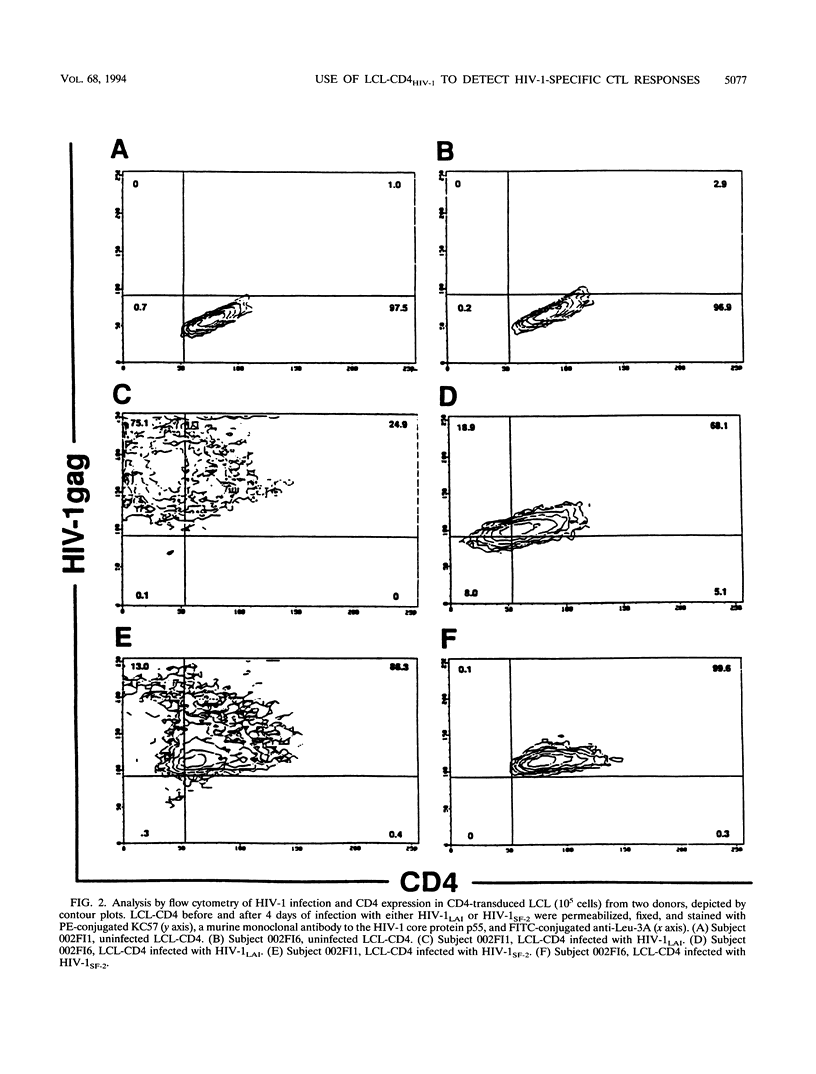
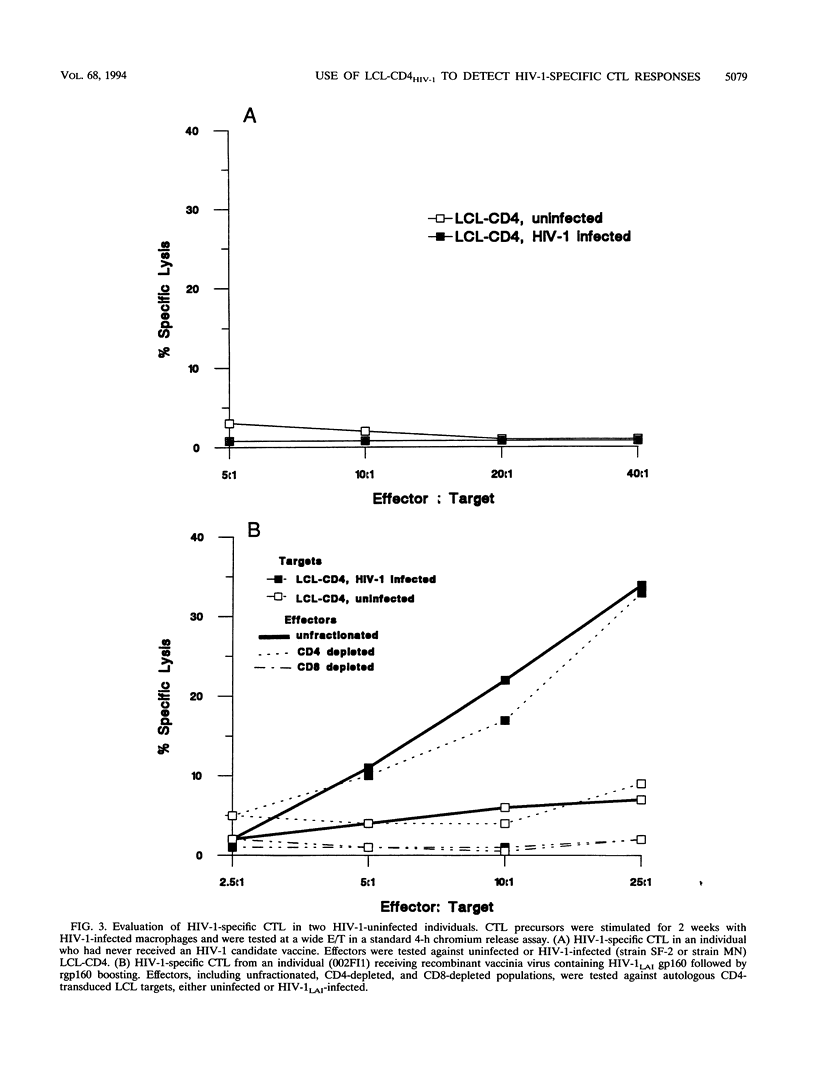
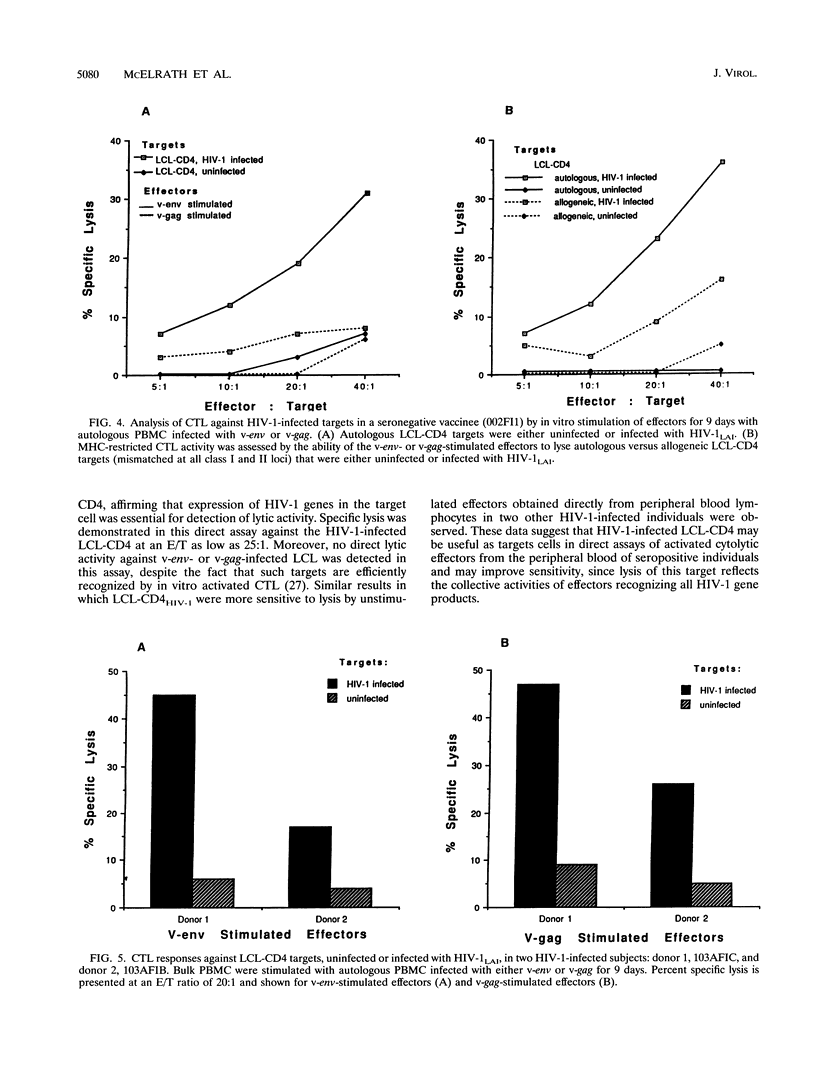
Abstract
Analysis of major histocompatibility complex-restricted cytotoxic T lymphocytes (CTL) capable of killing human immunodeficiency virus type 1 (HIV-1)-infected targets is essential for elucidating the basis for HIV-1 disease progression and the potential efficacy of candidate vaccines. The use of primary CD4+ T cells with variable infectivity as targets for such studies has significant limitations, and immortal autologous cells with high levels of CD4 expression that can be consistently infected with HIV-1 would be of much greater utility. Therefore, we transduced Epstein-Barr-virus-transformed B-lymphoblastoid cell lines (LCL) with a retroviral vector, LT4SN, containing the human CD4 gene. Stable LCL in which more than 95% of cells expressed membrane CD4 were obtained. Aliquots were infected with HIV-1, and, after 4 to 7 days, nearly all of the cells contained cytoplasmic gag and produced high levels of p24 antigen. The ability of major histocompatibility complex-restricted CD8+ CTL to lyse such HIV-1-infected CD4-transduced LCL (LCL-CD4HIV-1) was evaluated. These autologous targets were lysed by CTL generated from an HIV-1-uninfected vaccinee over a broad range of effector-to-target ratios. Similarly, the LCL-CD4HIV-1 were efficiently lysed by fresh circulating CTL from HIV-1-infected individuals, as well as by CTL activated by in vitro stimulation. Both HIV-1 env- and gag-specific CTL effectors lysed LCL-CD4HIV-1, consistent with the cellular expression of both HIV-1 genes. The LCL-CD4HIV also functioned as stimulator cells, and thus are capable of amplifying CTL against multiple HIV-1 gene products in HIV-1-infected individuals. The ability to produce HIV-1-susceptible autologous immortalized cell lines that can be employed as target cells should enable a more detailed evaluation of vaccine-induced CTL against both homologous and disparate HIV-1 strains. Furthermore, the use of LCL-CD4HIV-1 should facilitate the analysis of the range of HIV-1 gene products recognized by CTL in seropositive persons.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Abrahamsson B., Nagy K., Aurelius E., Gaines H., Nyström G., Fenyö E. M. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS. 1990 Feb;4(2):107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- Arendrup M., Nielsen C., Hansen J. E., Pedersen C., Mathiesen L., Nielsen J. O. Autologous HIV-1 neutralizing antibodies: emergence of neutralization-resistant escape virus and subsequent development of escape virus neutralizing antibodies. J Acquir Immune Defic Syndr. 1992;5(3):303–307. [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Cooney E. L., Collier A. C., Greenberg P. D., Coombs R. W., Zarling J., Arditti D. E., Hoffman M. C., Hu S. L., Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991 Mar 9;337(8741):567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- Cooney E. L., McElrath M. J., Corey L., Hu S. L., Collier A. C., Arditti D., Hoffman M., Coombs R. W., Smith G. E., Greenberg P. D. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl K., Martin K., Miller G. Differences among human immunodeficiency virus strains in their capacities to induce cytolysis or persistent infection of a lymphoblastoid cell line immortalized by Epstein-Barr virus. J Virol. 1987 May;61(5):1602–1608. doi: 10.1128/jvi.61.5.1602-1608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Kirchhoff F., Czajak S. C., Sehgal P. K., Desrosiers R. C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992 Dec 18;258(5090):1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- De Rossi A., Roncella S., Calabro M. L., D'Andrea E., Pasti M., Panozzo M., Mammano F., Ferrarini M., Chieco-Bianchi L. Infection of Epstein-Barr virus-transformed lymphoblastoid B cells by the human immunodeficiency virus: evidence for a persistent and productive infection leading to B cell phenotypic changes. Eur J Immunol. 1990 Sep;20(9):2041–2049. doi: 10.1002/eji.1830200924. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Gallo R. C., Koenig S., Salk J., Purcell R. H. NIH conference. Development and evaluation of a vaccine for human immunodeficiency virus (HIV) infection. Ann Intern Med. 1989 Mar 1;110(5):373–385. doi: 10.7326/0003-4819-110-5-373. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gotch F. M., Nixon D. F., Alp N., McMichael A. J., Borysiewicz L. K. High frequency of memory and effector gag specific cytotoxic T lymphocytes in HIV seropositive individuals. Int Immunol. 1990;2(8):707–712. doi: 10.1093/intimm/2.8.707. [DOI] [PubMed] [Google Scholar]
- Graham B. S., Matthews T. J., Belshe R. B., Clements M. L., Dolin R., Wright P. F., Gorse G. J., Schwartz D. H., Keefer M. C., Bolognesi D. P. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naive adults. The NIAID AIDS Vaccine Clinical Trials Network. J Infect Dis. 1993 Mar;167(3):533–537. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- Guillon J. M., Autran B., Denis M., Fouret P., Plata F., Mayaud C. M., Akoun G. M. Human immunodeficiency virus-related lymphocytic alveolitis. Chest. 1988 Dec;94(6):1264–1270. doi: 10.1378/chest.94.6.1264. [DOI] [PubMed] [Google Scholar]
- Hammond S. A., Bollinger R. C., Stanhope P. E., Quinn T. C., Schwartz D., Clements M. L., Siliciano R. F. Comparative clonal analysis of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ and CD8+ cytolytic T lymphocytes isolated from seronegative humans immunized with candidate HIV-1 vaccines. J Exp Med. 1992 Dec 1;176(6):1531–1542. doi: 10.1084/jem.176.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F. Scientific and social issues of human immunodeficiency virus vaccine development. Science. 1993 May 28;260(5112):1279–1286. doi: 10.1126/science.8493572. [DOI] [PubMed] [Google Scholar]
- Hoffenbach A., Langlade-Demoyen P., Dadaglio G., Vilmer E., Michel F., Mayaud C., Autran B., Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989 Jan 15;142(2):452–462. [PubMed] [Google Scholar]
- Hu S. L., Kosowski S. G., Dalrymple J. M. Expression of AIDS virus envelope gene in recombinant vaccinia viruses. Nature. 1986 Apr 10;320(6062):537–540. doi: 10.1038/320537a0. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Travis B. M., Garrigues J., Zarling J. M., Sridhar P., Dykers T., Eichberg J. W., Alpers C. Processing, assembly, and immunogenicity of human immunodeficiency virus core antigens expressed by recombinant vaccinia virus. Virology. 1990 Nov;179(1):321–329. doi: 10.1016/0042-6822(90)90300-g. [DOI] [PubMed] [Google Scholar]
- Kalams S. A., Johnson R. P., Trocha A. K., Dynan M. J., Ngo H. S., D'Aquila R. T., Kurnick J. T., Walker B. D. Longitudinal analysis of T cell receptor (TCR) gene usage by human immunodeficiency virus 1 envelope-specific cytotoxic T lymphocyte clones reveals a limited TCR repertoire. J Exp Med. 1994 Apr 1;179(4):1261–1271. doi: 10.1084/jem.179.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Hoth D. F. Development and testing of AIDS vaccines. Science. 1988 Jul 22;241(4864):426–432. doi: 10.1126/science.3293212. [DOI] [PubMed] [Google Scholar]
- Kundu S. K., Katzenstein D., Moses L. E., Merigan T. C. Enhancement of human immunodeficiency virus (HIV)-specific CD4+ and CD8+ cytotoxic T-lymphocyte activities in HIV-infected asymptomatic patients given recombinant gp160 vaccine. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11204–11208. doi: 10.1073/pnas.89.23.11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., McHugh T., Casavant C., Stites D., Oshiro L. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology. 1985 Dec;147(2):441–448. doi: 10.1016/0042-6822(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Montagnier L., Gruest J., Chamaret S., Dauguet C., Axler C., Guétard D., Nugeyre M. T., Barré-Sinoussi F., Chermann J. C., Brunet J. B. Adaptation of lymphadenopathy associated virus (LAV) to replication in EBV-transformed B lymphoblastoid cell lines. Science. 1984 Jul 6;225(4657):63–66. doi: 10.1126/science.6328661. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Klein G. Phenotypic and cytogenetic characteristics of human B-lymphoid cell lines and their relevance for the etiology of Burkitt's lymphoma. Adv Cancer Res. 1982;37:319–380. doi: 10.1016/s0065-230x(08)60886-6. [DOI] [PubMed] [Google Scholar]
- Orentas R. J., Hildreth J. E., Obah E., Polydefkis M., Smith G. E., Clements M. L., Siliciano R. F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp160 subunit vaccine. Science. 1990 Jun 8;248(4960):1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- Phillips R. E., Rowland-Jones S., Nixon D. F., Gotch F. M., Edwards J. P., Ogunlesi A. O., Elvin J. G., Rothbard J. A., Bangham C. R., Rizza C. R. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991 Dec 12;354(6353):453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- Richman D., Shih C. K., Lowy I., Rose J., Prodanovich P., Goff S., Griffin J. Human immunodeficiency virus type 1 mutants resistant to nonnucleoside inhibitors of reverse transcriptase arise in tissue culture. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11241–11245. doi: 10.1073/pnas.88.24.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere Y., Tanneau-Salvadori F., Regnault A., Lopez O., Sansonetti P., Guy B., Kieny M. P., Fournel J. J., Montagnier L. Human immunodeficiency virus-specific cytotoxic responses of seropositive individuals: distinct types of effector cells mediate killing of targets expressing gag and env proteins. J Virol. 1989 May;63(5):2270–2277. doi: 10.1128/jvi.63.5.2270-2277.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Hunter E. A., Gonda M. A., Sturzenegger S., Markham P. D., Gallo R. C. HTLV-III infection of EBV-genome-positive B-lymphoid cells with or without detectable T4 antigens. Int J Cancer. 1987 Feb 15;39(2):198–202. doi: 10.1002/ijc.2910390213. [DOI] [PubMed] [Google Scholar]
- Sugden B., Mark W. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J Virol. 1977 Sep;23(3):503–508. doi: 10.1128/jvi.23.3.503-508.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V., Britton S., Ehrnst A., Lenkei R., Strannegård O. Persistent productive HIV infection in EBV-transformed B lymphocytes. J Med Virol. 1989 Jan;27(1):19–24. doi: 10.1002/jmv.1890270105. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]



