Abstract
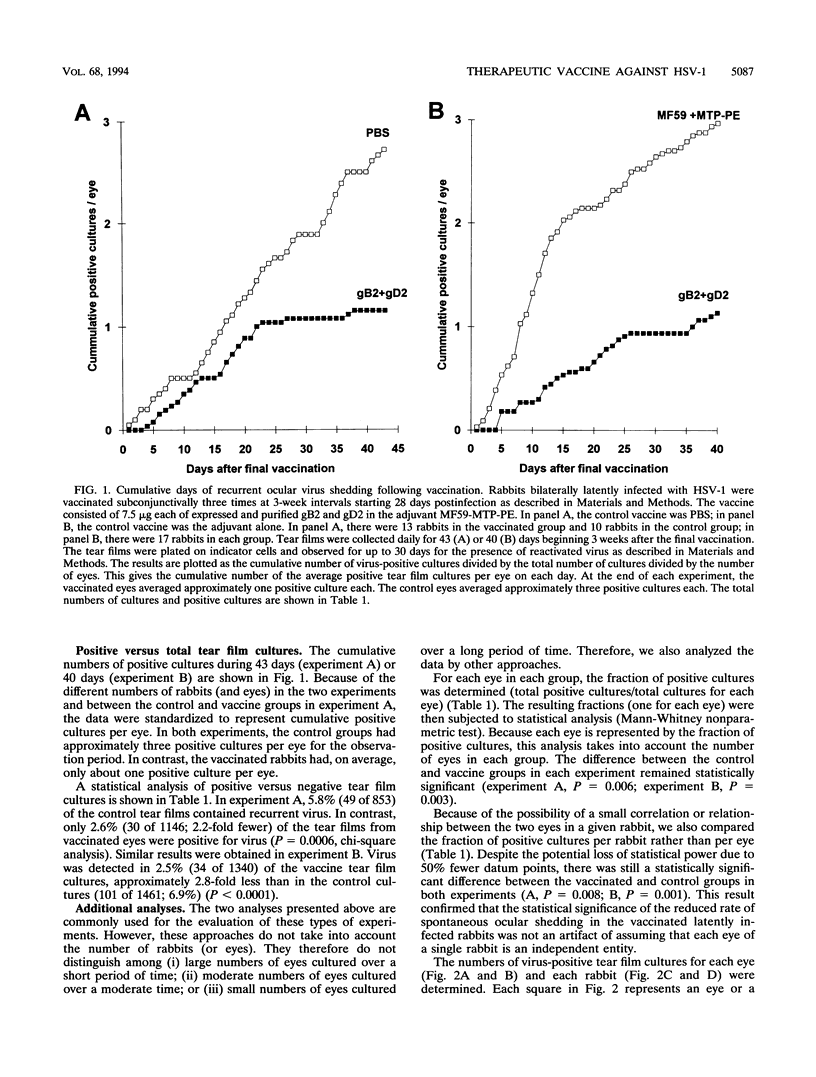
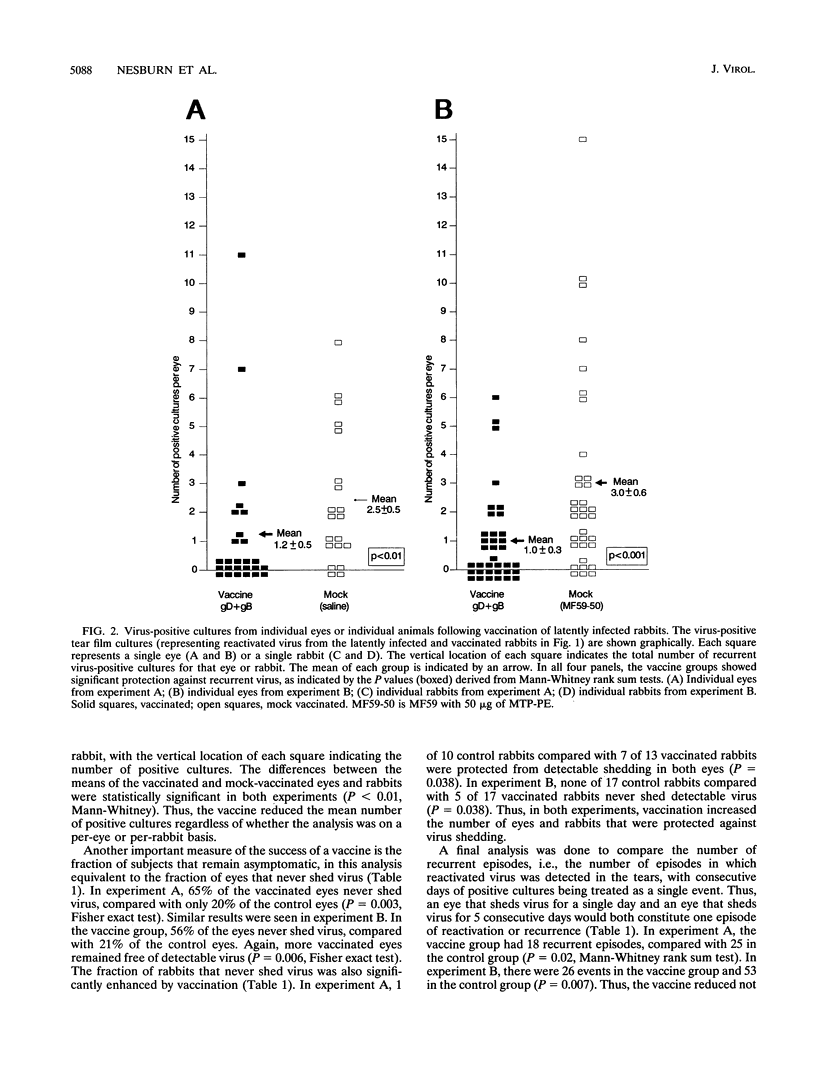
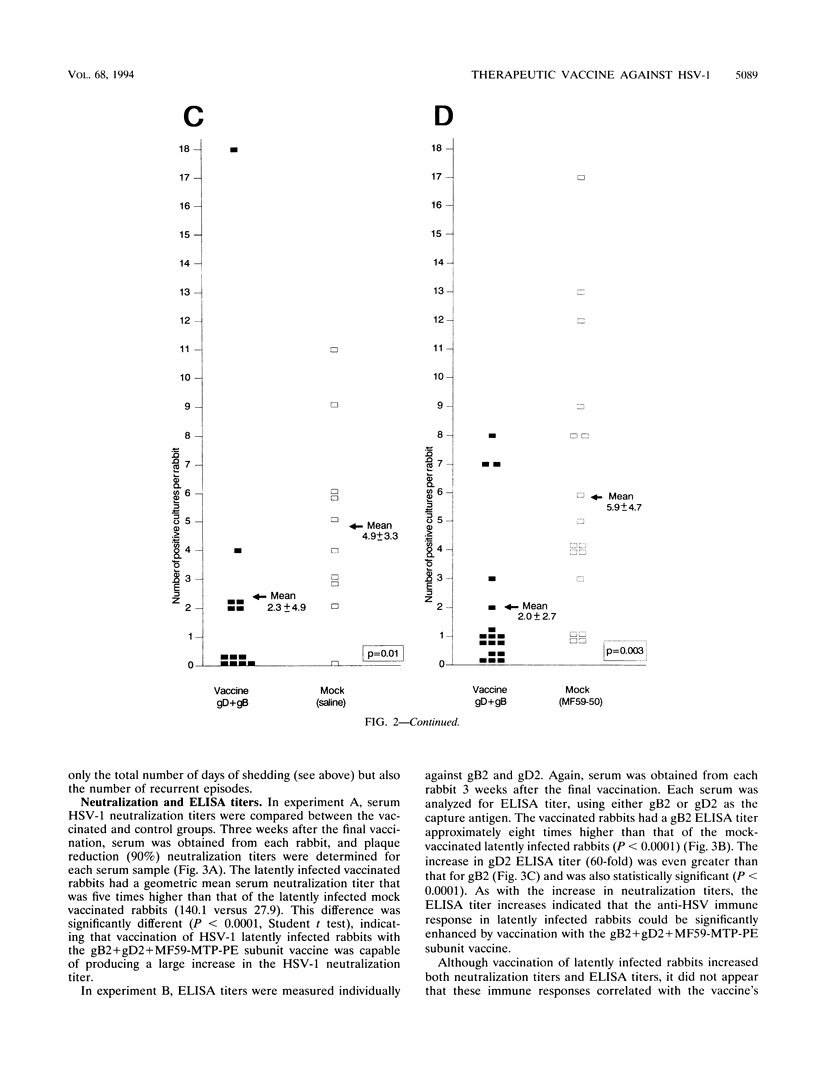
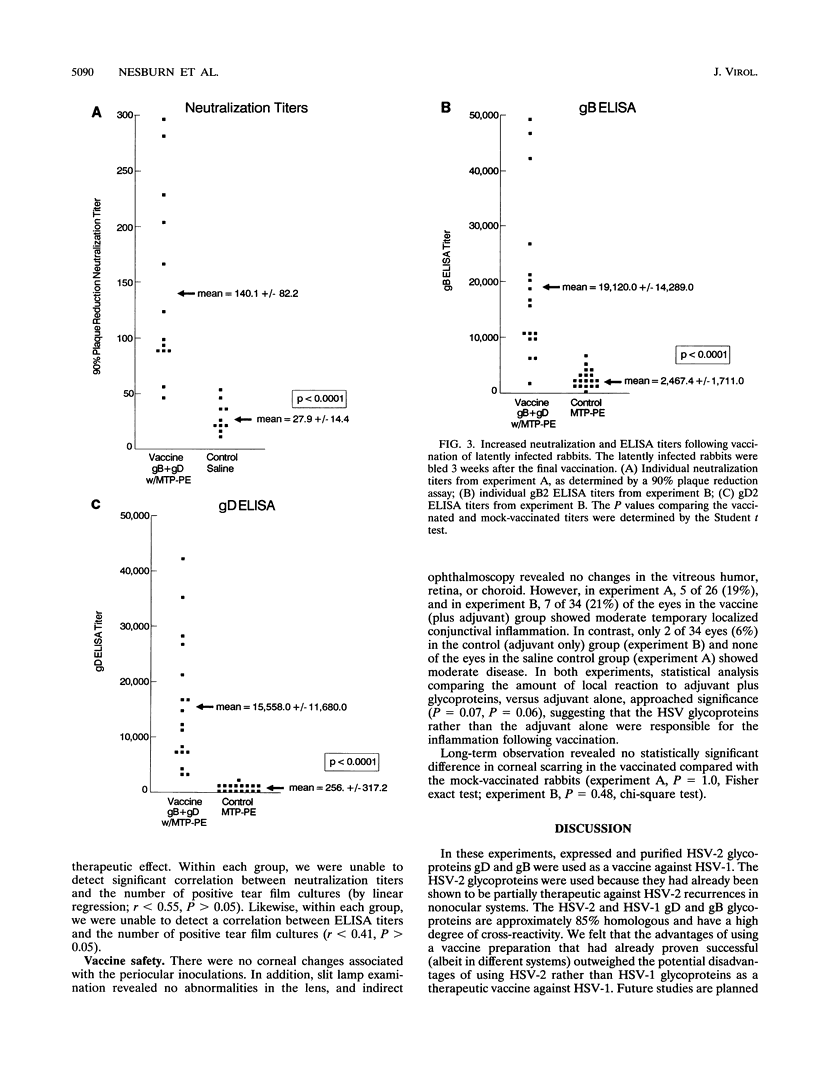
Periocular vaccination of rabbits with preexisting herpes simplex virus type 1 (HSV-1) latent infection with recombinant HSV-2 glycoproteins B and D (gB2 and gD2) plus adjuvant significantly reduced ocular viral shedding. Rabbits were infected in both eyes with HSV-1 strain McKrae. Following HSV-1 infection and the establishment of latency (28 days postinfection), rabbits were given a periocular subconjunctival vaccination three times at 3-week intervals. Beginning 3 weeks after the final vaccination, tear films were collected daily and cultured to detect the presence of HSV-1 and determine the spontaneous HSV-1 ocular shedding rates. Periocular vaccination increased the mean HSV-1 serum neutralizing antibody titer to fivefold above that seen in mock-vaccinated latently infected rabbits. gB enzyme-linked immunosorbent assay (ELISA) antibody titers were increased approximately 8-fold, and gD ELISA antibody titers were increased 60-fold. These increases were all statistically significant (P < 0.0001). In two independent experiments, vaccination reduced the spontaneous shedding rate by approximately 2.5-fold (P < 0.0004). In addition, the percentage of eyes that never shed virus during the 6 week postvaccination test period increased threefold (20% in controls versus 60% in vaccinated animals; P < 0.007). These results show that spontaneous ocular shedding of HSV-1 in latently infected rabbits can be significantly reduced by local periocular vaccination. This is the first report in any animal model of a successful therapeutic vaccine against recurrent HSV-1 ocular shedding. These results support the concept that development of a therapeutic vaccine for ocular HSV-1 recurrence in humans is possible.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke D. S. Vaccine therapy for HIV: a historical review of the treatment of infectious diseases by active specific immunization with microbe-derived antigens. Vaccine. 1993;11(9):883–891. doi: 10.1016/0264-410x(93)90374-7. [DOI] [PubMed] [Google Scholar]
- Burke R. L. Contemporary approaches to vaccination against herpes simplex virus. Curr Top Microbiol Immunol. 1992;179:137–158. doi: 10.1007/978-3-642-77247-4_9. [DOI] [PubMed] [Google Scholar]
- Corey L., Spear P. G. Infections with herpes simplex viruses (1). N Engl J Med. 1986 Mar 13;314(11):686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- Corey L., Spear P. G. Infections with herpes simplex viruses (2). N Engl J Med. 1986 Mar 20;314(12):749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Kaiwar R., Nesburn A. B., Slanina S., Wechsler S. L. Baculovirus-expressed glycoprotein E (gE) of herpes simplex virus type-1 (HSV-1) protects mice against lethal intraperitoneal and lethal ocular HSV-1 challenge. Virology. 1992 Jun;188(2):469–476. doi: 10.1016/0042-6822(92)90500-o. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Kaiwar R., Nesburn A. B., Wechsler S. L. Baculovirus expressed herpes simplex virus type 1 glycoprotein C protects mice from lethal HSV-1 infection. Antiviral Res. 1992 Jun;18(3-4):291–302. doi: 10.1016/0166-3542(92)90062-a. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Kaiwar R., Nesburn A. B., Wechsler S. L. Expression of herpes simplex virus type 1 glycoprotein B in insect cells. Initial analysis of its biochemical and immunological properties. Virus Res. 1992 Jan;22(1):25–39. doi: 10.1016/0168-1702(92)90087-p. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Kaiwar R., Nesburn A. B., Wechsler S. L. Expression of herpes simplex virus type 1 glycoprotein I in baculovirus: preliminary biochemical characterization and protection studies. J Virol. 1992 Apr;66(4):2505–2509. doi: 10.1128/jvi.66.4.2505-2509.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R. J., Burke R. L., Merigan T. C. Antigen-presenting liposomes are effective in treatment of recurrent herpes simplex virus genitalis in guinea pigs. J Virol. 1989 Jul;63(7):2951–2958. doi: 10.1128/jvi.63.7.2951-2958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R. J., Burke R. L., Merigan T. C. Liposome-formulated interleukin-2 as an adjuvant of recombinant HSV glycoprotein gD for the treatment of recurrent genital HSV-2 in guinea-pigs. Vaccine. 1992;10(4):209–213. doi: 10.1016/0264-410x(92)90153-b. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Incorvaia C., Di Luca D., Rotola A., Balboni P. G., Sebastiani A., Rossi A., Cassai E. Experimental keratitis in rabbits by human HSV-1 variants: prevention and treatment. J Med Virol. 1990 Nov;32(3):148–154. doi: 10.1002/jmv.1890320304. [DOI] [PubMed] [Google Scholar]
- Meignier B., Roizman B. Herpes simplex virus vaccines. Antiviral Res. 1985;Suppl 1:259–265. doi: 10.1016/s0166-3542(85)80036-x. [DOI] [PubMed] [Google Scholar]
- Mertz G. J., Ashley R., Burke R. L., Benedetti J., Critchlow C., Jones C. C., Corey L. Double-blind, placebo-controlled trial of a herpes simplex virus type 2 glycoprotein vaccine in persons at high risk for genital herpes infection. J Infect Dis. 1990 Apr;161(4):653–660. doi: 10.1093/infdis/161.4.653. [DOI] [PubMed] [Google Scholar]
- Nesburn A. B., Cook M. L., Stevens J. G. Latent herpes simplex virus. Isolation from rabbit trigeminal ganglia between episodes of recurrent ocular infection. Arch Ophthalmol. 1972 Oct;88(4):412–417. doi: 10.1001/archopht.1972.01000030414012. [DOI] [PubMed] [Google Scholar]
- Nesburn A. B., Dickinson R., Radnoti M., Green M. J. Experimental reactivation of ocular herpes simplex in rabbits. Surv Ophthalmol. 1976 Sep-Oct;21(2):185–190. doi: 10.1016/0039-6257(76)90098-9. [DOI] [PubMed] [Google Scholar]
- Nesburn A. B., Dunkel E. D., Trousdale M. D. Enhanced HSV recovery from neuronal tissues of latently infected rabbit. Proc Soc Exp Biol Med. 1980 Mar;163(3):398–401. doi: 10.3181/00379727-163-40785. [DOI] [PubMed] [Google Scholar]
- Nesburn A. B., Elliott J. H., Leibowitz H. M. Spontaneous reactivation of experimental herpes simplex keratitis in rabbits. Arch Ophthalmol. 1967 Oct;78(4):523–529. doi: 10.1001/archopht.1967.00980030525021. [DOI] [PubMed] [Google Scholar]
- Nesburn A. B., Robinson C., Dickinson R. Adenine arabinoside effect on experimental idoxuridine-resistant herpes simplex infection. Invest Ophthalmol. 1974 Apr;13(4):302–304. [PubMed] [Google Scholar]
- Newell C. K., Martin S., Sendele D., Mercadal C. M., Rouse B. T. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989 Feb;63(2):769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield R. R., Birx D. L., Ketter N., Tramont E., Polonis V., Davis C., Brundage J. F., Smith G., Johnson S., Fowler A. A phase I evaluation of the safety and immunogenicity of vaccination with recombinant gp160 in patients with early human immunodeficiency virus infection. Military Medical Consortium for Applied Retroviral Research. N Engl J Med. 1991 Jun 13;324(24):1677–1684. doi: 10.1056/NEJM199106133242401. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Nesburn A. B., Ghiasi H., Ong J., Lewis T. L., Lokensgard J. R., Wechsler S. L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Norley S., Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988 Jan-Feb;10(1):16–33. doi: 10.1093/clinids/10.1.16. [DOI] [PubMed] [Google Scholar]
- Salk J. Prospects for the control of AIDS by immunizing seropositive individuals. Nature. 1987 Jun 11;327(6122):473–476. doi: 10.1038/327473a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador L., Burke R. L., Ott G., Van Nest G. The effect of adjuvants on the efficacy of a recombinant herpes simplex virus glycoprotein vaccine. J Immunol. 1988 Sep 1;141(5):1720–1727. [PubMed] [Google Scholar]
- Shimomura Y., Gangarosa L. P., Sr, Kataoka M., Hill J. M. HSV-1 shedding by lontophoresis of 6-hydroxydopamine followed by topical epinephrine. Invest Ophthalmol Vis Sci. 1983 Dec;24(12):1588–1594. [PubMed] [Google Scholar]
- Smith R. E., McDonald H. R., Nesburn A. B., Minckler D. S. Penetrating keratoplasty: changing indications, 1947 to 1978. Arch Ophthalmol. 1980 Jul;98(7):1226–1229. doi: 10.1001/archopht.1980.01020040078009. [DOI] [PubMed] [Google Scholar]
- Stanberry L. R., Bernstein D. I., Burke R. L., Pachl C., Myers M. G. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987 May;155(5):914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- Stanberry L. R., Burke R., Myers M. G. Herpes simplex virus glycoprotein treatment of recurrent genital herpes. J Infect Dis. 1988 Jan;157(1):156–163. doi: 10.1093/infdis/157.1.156. [DOI] [PubMed] [Google Scholar]
- Stanberry L. R., Harrison C. J., Bernstein D. I., Burke R. L., Shukla R., Ott G., Myers M. G. Herpes simplex virus glycoprotein immunotherapy of recurrent genital herpes: factors influencing efficacy. Antiviral Res. 1989 May-Jun;11(4):203–214. doi: 10.1016/0166-3542(89)90005-3. [DOI] [PubMed] [Google Scholar]
- Stanberry L. R., Myers M. G., Stephanopoulos D. E., Burke R. L. Preinfection prophylaxis with herpes simplex virus glycoprotein immunogens: factors influencing efficacy. J Gen Virol. 1989 Dec;70(Pt 12):3177–3185. doi: 10.1099/0022-1317-70-12-3177. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Savarese B., Tigges M., Freifeld A. G., Krause P. R., Margolis D. M., Meier J. L., Paar D. P., Adair S. F., Dina D. Induction and enhancement of immune responses to herpes simplex virus type 2 in humans by use of a recombinant glycoprotein D vaccine. J Infect Dis. 1993 May;167(5):1045–1052. doi: 10.1093/infdis/167.5.1045. [DOI] [PubMed] [Google Scholar]
- Stuve L. L., Brown-Shimer S., Pachl C., Najarian R., Dina D., Burke R. L. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J Virol. 1987 Feb;61(2):326–335. doi: 10.1128/jvi.61.2.326-335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



