Abstract
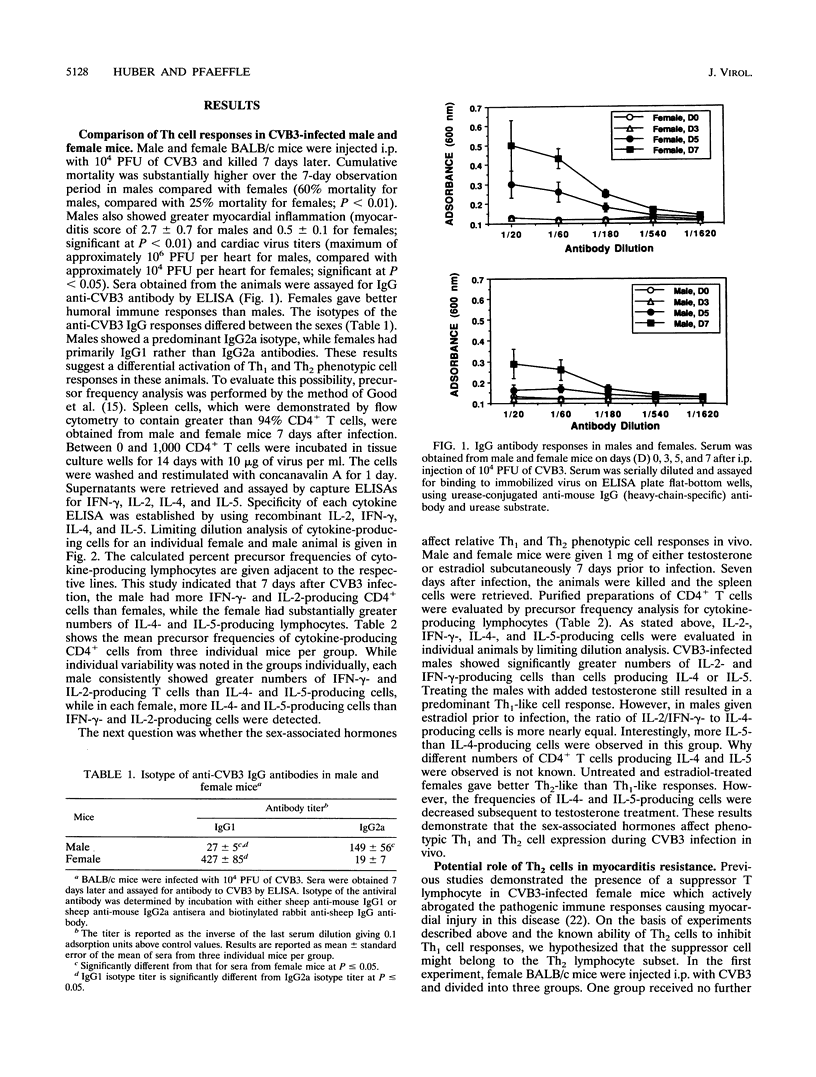
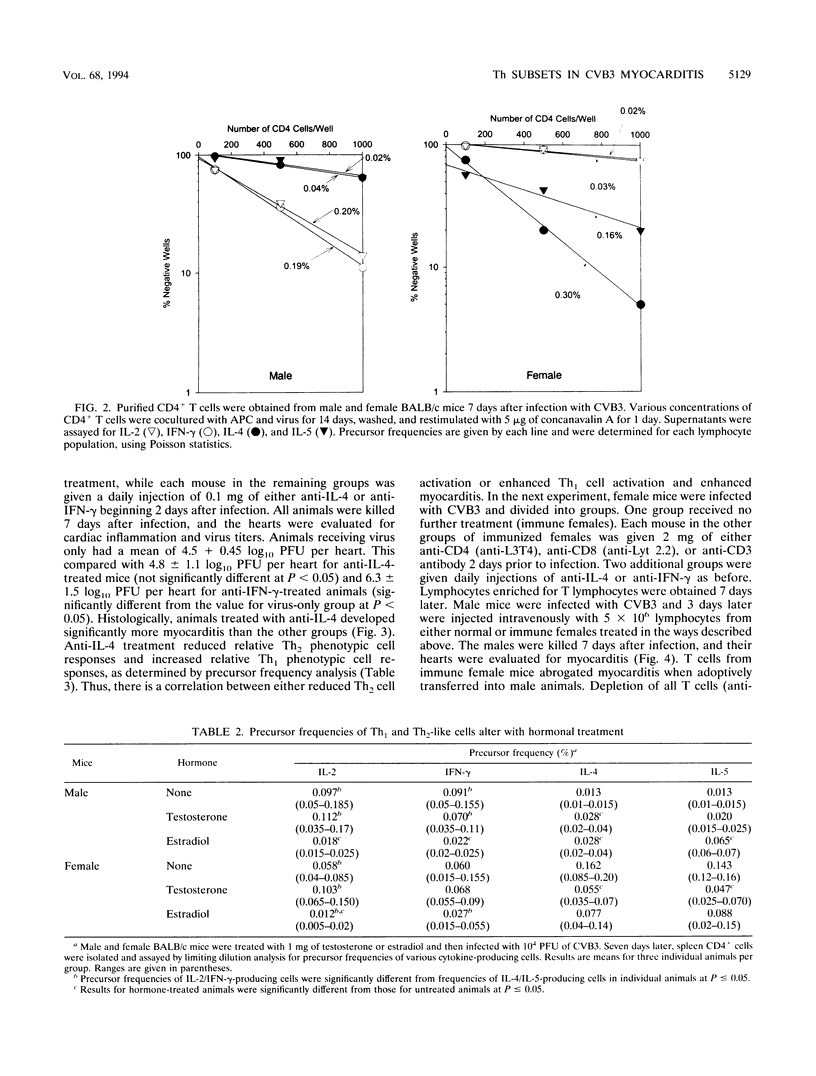
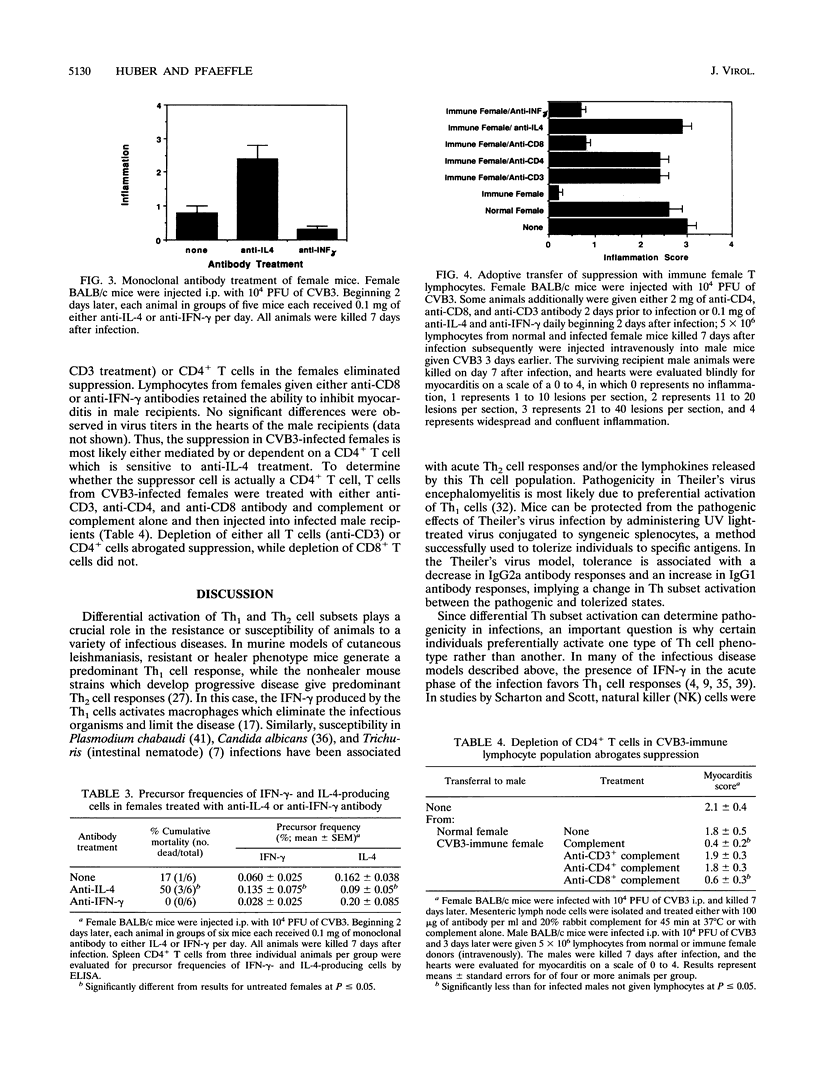
Male and female BALB/c mice differ dramatically in susceptibility to myocarditis subsequent to coxsackievirus B3 (CVB3) infection. CVB3 infection of male mice results in substantial inflammatory cell infiltration of the myocardium, and virus-immune lymphocytes from these animals give predominantly a Th1 cell phenotypic response, as determined by predominant immunoglobulin G2a isotypic antibody production and elevated numbers of gamma interferon and interleukin-2 (IL-2)-producing CD4+ T lymphocytes. Females infected with the same virus give predominantly a Th2 cell phenotypic response, as determined by preferential immunoglobulin G1 antibody isotypic responses and increased precursor frequencies of IL-4- and IL-5-producing CD4+ T cells. Treatment of females with testosterone or males with estradiol prior to infection alters subsequent Th subset differentiation, suggesting that the sex-associated hormones have either a direct or indirect effect on CD4+ lymphocyte responses in this model. Treatment of females with 0.1 mg of monoclonal antibody to IL-4 reduces precursor frequencies of IL-4-producing CD4+ T cells and increases frequencies of gamma interferon-producing cells. This treatment also enhances myocardial inflammation, indicating a correlation between Th1-like cell responses and pathogenicity in CVB3 infection. The Th2-like cell may regulate Th1 cell activation. Adoptive transfer of T lymphocytes from CVB3-infected female mice into male animals suppresses the development of myocarditis in the recipients. Treatment of the female donors with monoclonal antibodies to either CD3, CD4, or IL-4 molecules abrogates suppression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araneo B. A., Dowell T., Diegel M., Daynes R. A. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells. Blood. 1991 Aug 1;78(3):688–699. [PubMed] [Google Scholar]
- Bhalla A. K. Hormones and the immune response. Ann Rheum Dis. 1989 Jan;48(1):1–6. doi: 10.1136/ard.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Salgame P., Diamond B. Revisiting and revising suppressor T cells. Immunol Today. 1992 Apr;13(4):131–136. doi: 10.1016/0167-5699(92)90110-S. [DOI] [PubMed] [Google Scholar]
- Bogen S. A., Fogelman I., Abbas A. K. Analysis of IL-2, IL-4, and IFN-gamma-producing cells in situ during immune responses to protein antigens. J Immunol. 1993 May 15;150(10):4197–4205. [PubMed] [Google Scholar]
- Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993 Mar 19;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989 Dec;19(12):2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- Else K. J., Hültner L., Grencis R. K. Modulation of cytokine production and response phenotypes in murine trichuriasis. Parasite Immunol. 1992 Jul;14(4):441–449. doi: 10.1111/j.1365-3024.1992.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Esser C., Radbruch A. Immunoglobulin class switching: molecular and cellular analysis. Annu Rev Immunol. 1990;8:717–735. doi: 10.1146/annurev.iy.08.040190.003441. [DOI] [PubMed] [Google Scholar]
- Falcone V., Marelli P., Zolfino I., Campa M. BCG-activated NK cells regulate the antibody response to SRBC and restore immune reactivity to PPD in BCG-infected mice. Immunol Lett. 1993 Jun;36(3):295–299. doi: 10.1016/0165-2478(93)90103-9. [DOI] [PubMed] [Google Scholar]
- Fenoglio J. J., Jr, Ursell P. C., Kellogg C. F., Drusin R. E., Weiss M. B. Diagnosis and classification of myocarditis by endomyocardial biopsy. N Engl J Med. 1983 Jan 6;308(1):12–18. doi: 10.1056/NEJM198301063080103. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Fitch F. W., McKisic M. D., Lancki D. W., Gajewski T. F. Differential regulation of murine T lymphocyte subsets. Annu Rev Immunol. 1993;11:29–48. doi: 10.1146/annurev.iy.11.040193.000333. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Schell S. R., Nau G., Fitch F. W. Regulation of T-cell activation: differences among T-cell subsets. Immunol Rev. 1989 Oct;111:79–110. doi: 10.1111/j.1600-065x.1989.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Good M. F., Boyd A. W., Nossal G. J. Analysis of true anti-hapten cytotoxic clones in limit dilution microcultures after correction for "anti-self" activity: precursor frequencies, Ly-2 and Thy-1 phenotype, specificity, and statistical methods. J Immunol. 1983 May;130(5):2046–2055. [PubMed] [Google Scholar]
- Grossman C. J. Regulation of the immune system by sex steroids. Endocr Rev. 1984 Summer;5(3):435–455. doi: 10.1210/edrv-5-3-435. [DOI] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Auld K. R. Influence of sex hormones on Coxsackie B-3 virus infection in Balb/c mice. Cell Immunol. 1982 Feb;67(1):173–179. doi: 10.1016/0008-8749(82)90210-6. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Woodruff J. F. Sex-related differences in the pattern of coxsackievirus B-3-induced immune spleen cell cytotoxicity against virus-infected myofibers. Infect Immun. 1981 Apr;32(1):68–73. doi: 10.1128/iai.32.1.68-73.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984 Jul;116(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Job L. P., Lyden D. C., Huber S. A. Demonstration of suppressor cells in coxsackievirus group B, type 3 infected female Balb/c mice which prevent myocarditis. Cell Immunol. 1986 Mar;98(1):104–113. doi: 10.1016/0008-8749(86)90271-6. [DOI] [PubMed] [Google Scholar]
- Kendall C., Ionescu-Matiu I., Dreesman G. R. Utilization of the biotin/avidin system to amplify the sensitivity of the enzyme-linked immunosorbent assay (ELISA). J Immunol Methods. 1983 Feb 11;56(3):329–339. doi: 10.1016/s0022-1759(83)80022-2. [DOI] [PubMed] [Google Scholar]
- Lerner A., Wilson F. M., Reyes M. P. Enteroviruses and the heart (with special emphasis on the probable role of coxsackieviruses, group B, types 1-5). II. Observations in humans. Mod Concepts Cardiovasc Dis. 1975 Mar;44(3):11–15. [PubMed] [Google Scholar]
- Leslie K., Blay R., Haisch C., Lodge A., Weller A., Huber S. Clinical and experimental aspects of viral myocarditis. Clin Microbiol Rev. 1989 Apr;2(2):191–203. doi: 10.1128/cmr.2.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D. C., Olszewski J., Feran M., Job L. P., Huber S. A. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987 Mar;126(3):432–438. [PMC free article] [PubMed] [Google Scholar]
- Morris L., Troutt A. B., Handman E., Kelso A. Changes in the precursor frequencies of IL-4 and IFN-gamma secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol. 1992 Oct 15;149(8):2715–2721. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Oswald I. P., Gazzinelli R. T., Sher A., James S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992 Jun 1;148(11):3578–3582. [PubMed] [Google Scholar]
- Paavonen T. Hormonal regulation of lymphocyte functions. Med Biol. 1987;65(5-6):229–240. [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. D., Karpus W. J., Clatch R. J., Miller S. D. Split tolerance of Th1 and Th2 cells in tolerance to Theiler's murine encephalomyelitis virus. Eur J Immunol. 1993 Jan;23(1):46–55. doi: 10.1002/eji.1830230109. [DOI] [PubMed] [Google Scholar]
- Reyes M. P., Lerner A. M. Coxsackievirus myocarditis--with special reference to acute and chronic effects. Prog Cardiovasc Dis. 1985 May-Jun;27(6):373–394. doi: 10.1016/0033-0620(85)90001-5. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992 Oct;13(10):379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Cenci E., Spaccapelo R., Mosci P., Puccetti P., Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993 Feb 1;150(3):925–931. [PubMed] [Google Scholar]
- Romani L., Mencacci A., Cenci E., Spaccapelo R., Schiaffella E., Tonnetti L., Puccetti P., Bistoni F. Natural killer cells do not play a dominant role in CD4+ subset differentiation in Candida albicans-infected mice. Infect Immun. 1993 Sep;61(9):3769–3774. doi: 10.1128/iai.61.9.3769-3774.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röcken M., Saurat J. H., Hauser C. A common precursor for CD4+ T cells producing IL-2 or IL-4. J Immunol. 1992 Feb 15;148(4):1031–1036. [PubMed] [Google Scholar]
- Sarvetnick N., Fox H. S. Interferon-gamma and the sexual dimorphism of autoimmunity. Mol Biol Med. 1990 Aug;7(4):323–331. [PubMed] [Google Scholar]
- Scharton T. M., Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993 Aug 1;178(2):567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg A. D., Huston D. P., Taurog J. D., Cowdery J. S., Ravecheé E. S. The cellular and genetic basis of murine lupus. Immunol Rev. 1981;55:121–154. doi: 10.1111/j.1600-065x.1981.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol. 1993 Apr;92(1):77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Styrt B., Sugarman B. Estrogens and infection. Rev Infect Dis. 1991 Nov-Dec;13(6):1139–1150. doi: 10.1093/clinids/13.6.1139. [DOI] [PubMed] [Google Scholar]
- Thomas J. M., Carver F. M., Cunningham P., Olsen L., Thomas F. T. Veto cells induce long-term kidney allograft tolerance in primates without chronic immunosuppression. Transplant Proc. 1991 Feb;23(1 Pt 1):11–13. [PubMed] [Google Scholar]
- Torbett B. E., Laxer J. A., Glasebrook A. L. Frequencies of T cells secreting IL-2 and/or IL-4 among unprimed CD4+ populations. Evidence that clones secreting IL-2 and IL-4 give rise to clones which secrete only IL-4. Immunol Lett. 1990 Jan;23(3):227–233. doi: 10.1016/0165-2478(90)90197-x. [DOI] [PubMed] [Google Scholar]
- Varkila K., Chatelain R., Leal L. M., Coffman R. L. Reconstitution of C.B-17 scid mice with BALB/c T cells initiates a T helper type-1 response and renders them capable of healing Leishmania major infection. Eur J Immunol. 1993 Jan;23(1):262–268. doi: 10.1002/eji.1830230141. [DOI] [PubMed] [Google Scholar]
- Weaver C. T., Hawrylowicz C. M., Unanue E. R. T helper cell subsets require the expression of distinct costimulatory signals by antigen-presenting cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8181–8185. doi: 10.1073/pnas.85.21.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A. H., Simpson K., Herzum M., Van Houten N., Huber S. A. Coxsackievirus-B3-induced myocarditis: virus receptor antibodies modulate myocarditis. J Immunol. 1989 Sep 15;143(6):1843–1850. [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. III. Role of sex. J Immunol. 1977 Aug;119(2):591–597. [PubMed] [Google Scholar]
- Woodruff J. F. Viral myocarditis. A review. Am J Pathol. 1980 Nov;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]
- Yamamoto M., Fujihashi K., Beagley K. W., McGhee J. R., Kiyono H. Cytokine synthesis by intestinal intraepithelial lymphocytes. Both gamma/delta T cell receptor-positive and alpha/beta T cell receptor-positive T cells in the G1 phase of cell cycle produce IFN-gamma and IL-5. J Immunol. 1993 Jan 1;150(1):106–114. [PubMed] [Google Scholar]


