Summary
Olfactory neurons project their axons to spatially-invariant glomeruli in the olfactory bulb, forming an ordered pattern of innervation comprising the olfactory sensory map. A mirror symmetry exists within this map, such that neurons expressing a given receptor typically project to one glomerulus on the medial face and one glomerulus on the lateral face of the bulb. The mechanisms underlying an olfactory neuron’s choice to project medially vs. laterally remain largely unknown, however. Here we demonstrate that insulin-like growth factor (IGF) signaling is required for sensory innervation of the lateral olfactory bulb. Mutations that eliminate IGF signaling cause axons destined for targets in the lateral bulb to shift to ectopic sites on the ventral-medial surface. Using primary cultures of olfactory and cerebellar neurons we further show that IGF is a chemoattractant for axon growth cones. Together these observations reveal a role of IGF signaling in sensory map formation and axon guidance.
Introduction
In sensory systems, ordered patterns of neuronal connections represent information about complex stimuli from the external world. In the visual system, for example, retinal ganglion cells form a point-to-point topographic map in their projection to thalamic or midbrain structures, representing information about the position of the stimulus in visual space (McLaughlin and O'Leary, 2005). Similarly, sensory neurons in the somatosensory or auditory systems maintain neighbor relationships when projecting to the thalamus, resulting in a spatial map of the body surface or sound frequency, respectively (Killackey et al., 1995; Rubel and Fritzsch, 2002). In contrast, the projection of primary olfactory sensory neurons to their first relay in the brain comprises a discontinuous map; neurons expressing a given odorant receptor, while distributed broadly in the peripheral sensory epithelium (Ressler et al., 1993; Vassar et al., 1993) converge to discrete and spatially invariant glomeruli in the olfactory bulb (Mombaerts et al., 1996; Mori et al., 2006; Ressler et al., 1994; Vassar et al., 1994). The pattern of these convergent connections forms the anatomical basis for the olfactory sensory map. This sensory map displays a mirror symmetry, such that odorant receptor-specific neurons typically extend their axons to one glomerulus in the medial hemisphere and another glomerulus in the lateral hemisphere of the olfactory bulb (Mombaerts et al., 1996; Nagao et al., 2000; Ressler et al., 1994; Vassar et al., 1994).
How is the olfactory sensory map established during development? Olfactory neurons express just one odorant receptor allele from a repertoire of ~1000 odorant receptor genes (Chess et al., 1994). The selection of an odorant receptor gene not only determines the specificity of the cell for odorants, but also influences the targeting of its axon in the olfactory bulb (Feinstein and Mombaerts, 2004; Imai et al., 2006; Mombaerts et al., 1996; Wang et al., 1998). While the odorant receptor plays a critical role in determining the projection pattern of olfactory sensory axons, it does not appear to be the sole determinant of glomerular position (Imai et al., 2006; Mombaerts et al., 1996; Wang et al., 1998). Thus the formation of the olfactory sensory map is thought to involve a hierarchy of cues and axon guidance decisions, which together function to direct the axons of neurons expressing the same odorant receptor to common targets in the olfactory bulb (Lin and Ngai, 1999; St John et al., 2002). However, numerous studies addressing the role of classical axon guidance cues and cell adhesion molecules in the developing olfactory system have yet to provide a complete understanding of how the stereotyped glomerular map is formed with such precision (Cho et al., 2007; Cutforth et al., 2003; Imai et al., 2006; Montag-Sallaz et al., 2002; Puche et al., 1996; Schwarting et al., 2000; Serizawa et al., 2006; Tisay et al., 2000; Treloar et al., 1997; Walz et al., 2006; Walz et al., 2002) (reviewed in Imai and Sakano, 2007; Mombaerts, 2006).
In the present study, we examined the role of insulin-like growth factor (IGF) signaling in axon guidance using the developing olfactory system as our primary model. The IGF family of signaling effectors includes two secreted polypeptide ligands, IGF1 and IGF2, which bind to and activate a common receptor tyrosine kinase, the type 1 IGF receptor (IGF1R) (Efstratiadis, 1998). Developmentally, IGF signaling plays an important role in determining body size by promoting cell proliferation and survival (Efstratiadis, 1998); null mutations in Igf1, Igf2 or Igf1r in mice result in severe growth deficiency phenotypes (Baker et al., 1993; DeChiara et al., 1990; Liu et al., 1993). In the nervous system, IGF signaling apparently mediates neuronal survival and proliferation (Beck et al., 1995; Chrysis et al., 2001; Ye et al., 1996). In addition, IGF signaling has recently been shown to regulate growth cone expansion (Laurino et al., 2005), promote axon outgrowth in corticospinal neurons (Ozdinler and Macklis, 2006), and play a role in establishing neuronal polarity in cultured hippocampal neurons (Sosa et al., 2006). Thus, a role for IGF signaling in neuronal patterning is beginning to emerge that is distinct from the classical view of IGFs as growth promoting factors. It remains unclear, however, whether IGFs play a wider role in neuronal patterning or serve as instructive cues affecting axon guidance and axon targeting decisions.
Here we describe results indicating that both IGF1 and IGF2 are expressed by cells in and surrounding the olfactory bulb, whereas IGF1R is expressed in a complementary fashion on olfactory sensory axons. Using a series of IGF mutant mice we show that the loss of IGF signaling causes a severe misrouting of olfactory sensory axons away from the lateral olfactory bulb to ectopic ventral-medial positions, resulting in a dramatic perturbation of the mirror-symmetric olfactory sensory map. We further demonstrate that IGF1 can act as a chemoattractant for growth cones of cultured olfactory neurons as well as cultured cerebellar granule neurons. These data demonstrate the importance of IGF signaling in the formation of the olfactory sensory map, and suggest a general role of IGFs as instructive axon guidance cues.
Results
IGF Ligands and their Receptor are Expressed in Complementary Patterns in the Developing Olfactory System
We previously performed a microarray-based screen to identify candidate genes expressed in the olfactory bulb that could act to guide olfactory axons to their target glomeruli (Lin et al., 2004). One candidate signaling pathway identified by this screen was the IGF signaling pathway. To characterize further the IGF signaling components in the olfactory system, we performed immunohistochemistry on the developing olfactory system at embryonic (E) day 14.5 and 18.5 for IGF1, IGF2 and IGF1R. At E14.5, IGF1 is expressed circumferentially in the developing bulb, just within and in close apposition to the developing olfactory nerve layer (Figure 1A). Interestingly, at rostral positions there appears to be a gradient of IGF1 along the medial-lateral axis, such that expression is higher in the lateral portion of the olfactory bulb. This gradient of expression diminishes and eventually reverses to display a medial > lateral bias more caudally. Localization of olfactory marker protein (OMP), a marker for all olfactory neurons and their axons, reveals a corresponding pattern of olfactory sensory innervation at this stage of development. The medial-lateral gradient of IGF1 expression is not readily detectable at E15.5 (data not shown), and by E18.5 IGF1 localizes to the mitral cell and glomerular layers (which are more distinct at this stage) throughout the olfactory bulb (Figure 1B).
Figure 1. IGF Signaling Components Are Expressed in the Developing Olfactory System.
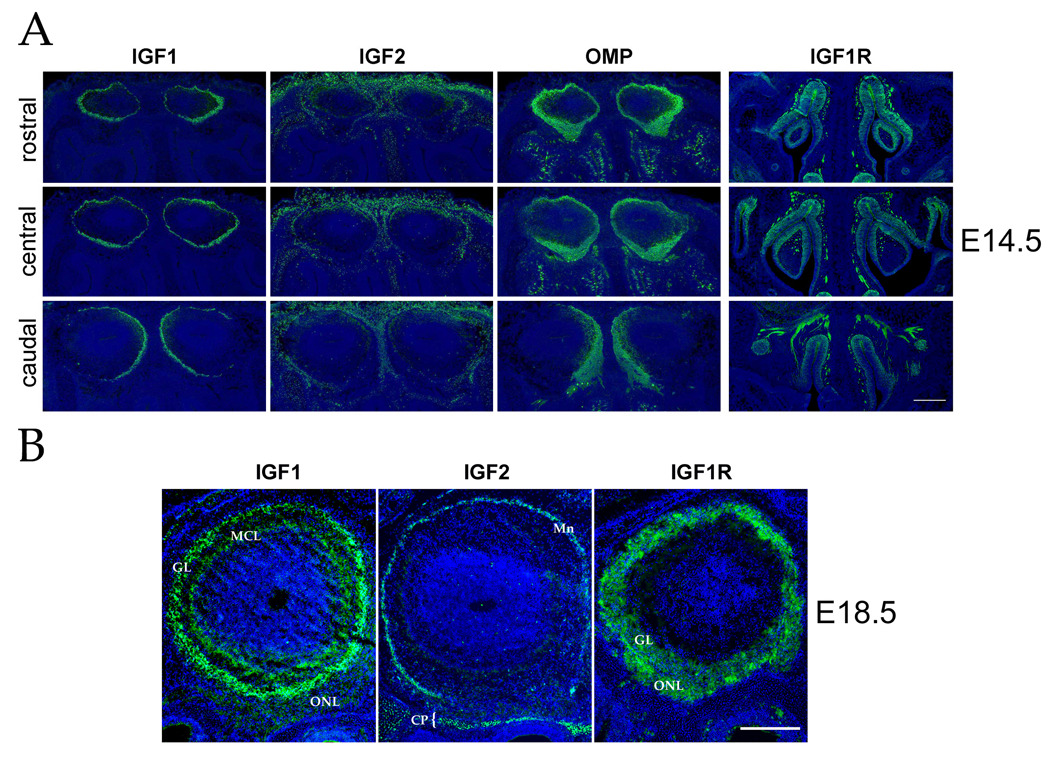
(A) Representative coronal tissue sections from the rostral, center, and caudal regions of the olfactory bulb and epithelium of E14.5 mice were labeled with IGF1, IGF2, IGF1R, or olfactory marker protein (OMP) antibodies. IGF1, IGF2 and OMP immunoreactivity (green) is shown from representative adjacent tissue sections taken at each position along the rostral-caudal axis. At this stage, IGF1 localizes in each bulb immediately inside the olfactory nerve layer (likely in the developing mitral cell and glomerular layers), showing a slight lateral > medial bias in expression rostrally and a pronounced medial > lateral bias caudally. IGF2 immunoreactivity is observed both in the overlying skin as well as in the leptomeninges of the olfactory bulb, with an exclusion from the ventral bulb. OMP expression – reflecting the disposition of the olfactory sensory axons in the olfactory nerve layer – reveals innervation roughly approximating the location of IGF1 in underlying bulb structures. In the olfactory epithelium, IGF1R is localized to immature and mature olfactory neurons throughout the olfactory sensory epithelium, as well as in the underlying olfactory axon fascicles (see Figure S1). (B) Coronal tissue sections of olfactory bulbs from E18.5 animals were analyzed by immunohistochemistry for IGF1, IGF2 and IGF1R expression. IGF1 is expressed in the mitral cell layer (MCL) and glomerular layer (GL) of the olfactory bulb. IGF2 is found in the leptomeninges (Mn) surrounding the bulb as well as in the bone of the cribriform plate (CP and bracket). Note that the expression of IGF2 in the leptomeninges does not completely surround the bulb in this tissue section. IGF1R localizes to the olfactory neuron axons within the olfactory nerve layer (ONL) as well as in the glomerular layer (GL). Nuclei of cells were visualized with Hoechst 33342 (blue). Dorsal is to the top in all panels; medial is to the right in panel B. Scale bar = 200 µm in panel A and 100 µm in panel B.
Consistent with previous RNA in situ hybridization results (Lin et al., 2004; Stylianopoulou et al., 1988), IGF2 protein is expressed in the leptomeninges surrounding the bulb. The highest expression is in the dorsal region (seen as diffuse staining at E14.5, becoming more discrete by E18.5), whereas expression is diminished in the rostral-ventral region (Figure 1A and B). Cells of the cribriform plate also express IGF2 (bracket in Figure 1B), where it may interact with olfactory sensory axons as they traverse this structure en route to the olfactory bulb. Together these observations confirm that the two known IGF ligands are expressed in locations in which they could interact with olfactory sensory axons entering the olfactory bulb.
In order for olfactory neurons to respond to olfactory bulb-derived IGF signals, these cells must express IGF1R, the receptor mediating both IGF1 and IGF2 signaling. Similar to previous observations in the rat olfactory epithelium (Ferrari et al., 2003; Suzuki and Takeda, 2002), at E14.5 we found IGF1R to be expressed in immature and mature olfactory neurons, as well as in the underlying sensory axon fascicles (Figure S1), showing no obvious restrictions along the three principle axes (medial-lateral, dorsal-ventral, or rostral-caudal) of the olfactory epithelium (Figure 1A). IGF1R is also present within the olfactory bulb nerve layer at E14.5 (data not shown), and IGF1R-positive axons can be observed entering the glomerular layer by E18.5 (Figure 1B). Thus, the olfactory sensory axons express IGF1R, which presumably makes them able to respond to IGF cues found in the olfactory bulb as they grow into and innervate this structure.
IGF Signaling Is Required for the Normal Projection of Olfactory Sensory Axons in the Olfactory Bulb
The complementary expression of IGF ligands in the olfactory bulb and IGF1R in olfactory sensory axons suggests a role for IGF signaling in the projection of primary sensory axons in the olfactory bulb. We tested this hypothesis by evaluating the projection patterns of olfactory sensory neurons in mice harboring a targeted deletion in the Igf1r gene (Liu et al., 1993). To facilitate the visualization of olfactory sensory axons, we examined mice expressing a tau:lacZ fusion protein in all olfactory neurons under the control of the olfactory marker protein promoter (Mombaerts et al., 1996). The innervation of the olfactory bulb by olfactory neurons was visualized by immunohistochemical staining using an anti-β-galactosidase antibody. Because homozygous Igf1r null mutants die immediately after birth (Liu et al., 1993), the olfactory projection pattern was assessed at embryonic stages, when some, but not all glomeruli have formed. Staining for β-galactosidase in coronal tissue sections from embryos as early as E15.5 demonstrates a striking defect in Igf1r−/− mice compared to control Igf1r+/+ or Igf1r+/− littermates. Whereas olfactory axons in control mice project to and innervate the entire circumference of the olfactory bulb, homozygous mutant mice show a marked decrease in innervation the lateral face of each bulb (compare Figure 2A and 2B). Similar results were observed at E18.5 (compare Figure 2C and 2D), as well as at E16.5 and E17.5 (data not shown). Analysis of serial tissue sections collected along the rostral-caudal axis reveals the paucity of lateral innervation throughout the olfactory bulb in Igf1r−/− mice, although this effect is somewhat less pronounced at rostral-most positions (Figure S2). By contrast, in wild type animals the entire circumference of the olfactory bulb is innervated, with a slight bias toward the lateral face in the rostral bulb (Figure S2).
Figure 2. Innervation of the Lateral Olfactory Bulb Is Severely Reduced in the Absence of IGF Signaling.
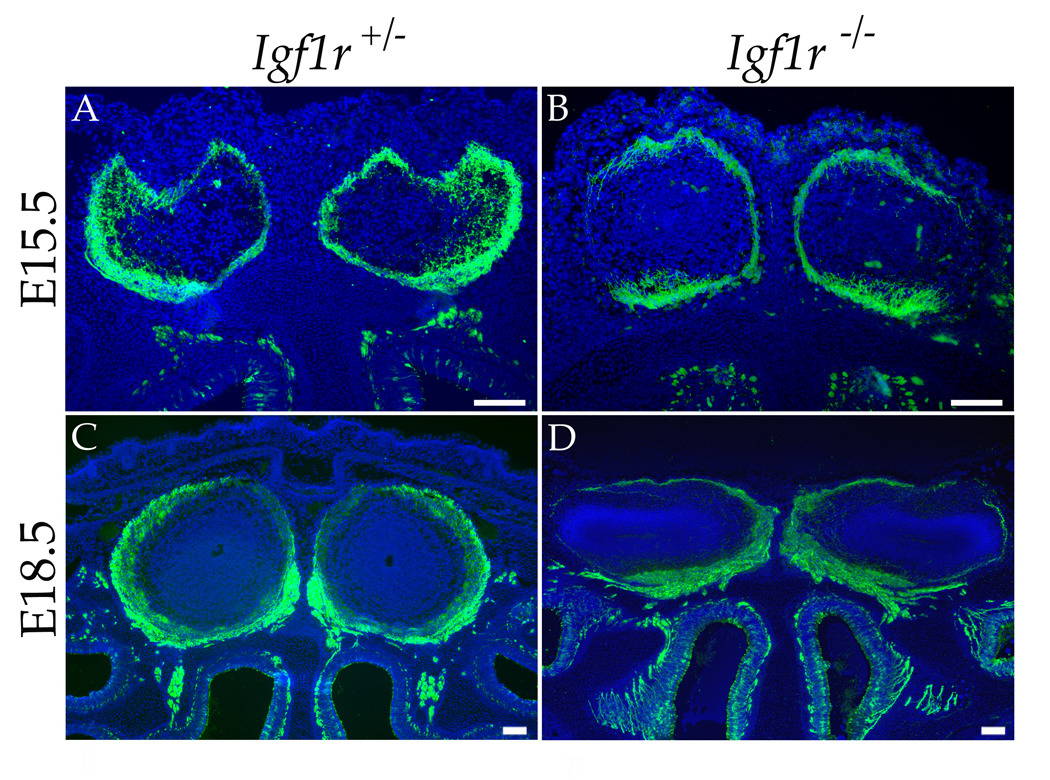
Anti-β-galactosidase staining (green) of coronal sections from heads of OMP-IRES-tau:lacZ; Igf1r+/− (A and C) and OMP-IRES-tau:lacZ; Igf1r−/− (B and D) mice reveal a deficiency in olfactory axon targeting to the lateral face of the olfactory bulb during development of the Igf1r−/− animals. (A, B) E15.5 animals show that at this early stage of olfactory map formation, the absence of IGF signaling perturbs the symmetry of ORN projections to the medial and lateral olfactory bulb (compare A [heterozygous control] to B [Igf1r null mutant]). (C,D) A comparison at E18.5 shows a similar lack of innervation of the lateral face of the olfactory bulb in the Igf1r null mutant (D) compared to the control (C). Nuclei were stained with Hoechst 33342 (blue). Scale bar = 100µm.
The appearance of the observed innervation defect relatively early in the development of the olfactory projection (E15.5) suggests that IGF signaling is required for the initial innervation of the lateral olfactory bulb. The phenotype appeared to be fully penetrant and was observed in 2/2 mutants at E15.5 (compared to 3 heterozygous control littermates), 2/2 mutants at E16.5 (2 heterozygous controls), 1/1 mutant at E17.5 (2 heterozygote controls), and 13/13 mutants at E18.5 (6 wild type or heterozygous controls). In Igf1r−/− mice examined at more advanced embryonic stages, the olfactory bulbs appeared to be somewhat compressed along the dorsal-ventral axis (Figure 2D and Figure S2). Nonetheless, the dramatic decrease of lateral innervation remained a consistent feature in mutant olfactory bulbs, with the olfactory sensory axons appearing to accumulate along the ventral-medial region of the olfactory bulb.
Compensation of Olfactory Bulb Innervation Defects in Igf1 or Igf2 Mutants
The results described above demonstrate the necessity of IGF signaling for the innervation of the lateral face of the olfactory bulb by olfactory sensory axons. We next wished to determine which of the two IGF ligands is responsible for directing the axons to the lateral olfactory bulb. Based on the lateral > medial bias in IGF1 expression observed in the olfactory bulb at E14.5, it seemed plausible that IGF1 could be required for the innervation of the lateral olfactory bulb. To test this idea, we examined the projection of olfactory neurons in bulbs from mutant mice lacking either the Igf1 or Igf2 gene. Olfactory axons were localized using an anti-OMP antibody in Igf1−/−, Igf2−/−, or heterozygous littermate controls at E18.5 (Figure 3). In both Igf1 and Igf2 single null mutants the pattern of sensory axon innervation in the olfactory bulb was essentially indistinguishable from controls (n = 5 mice for Igf1−/−; n = 2 for Igf2−/−).
Figure 3. Effects of Targeted Mutations in the Igf1 and Igf2 Genes on Olfactory Bulb Innervation.
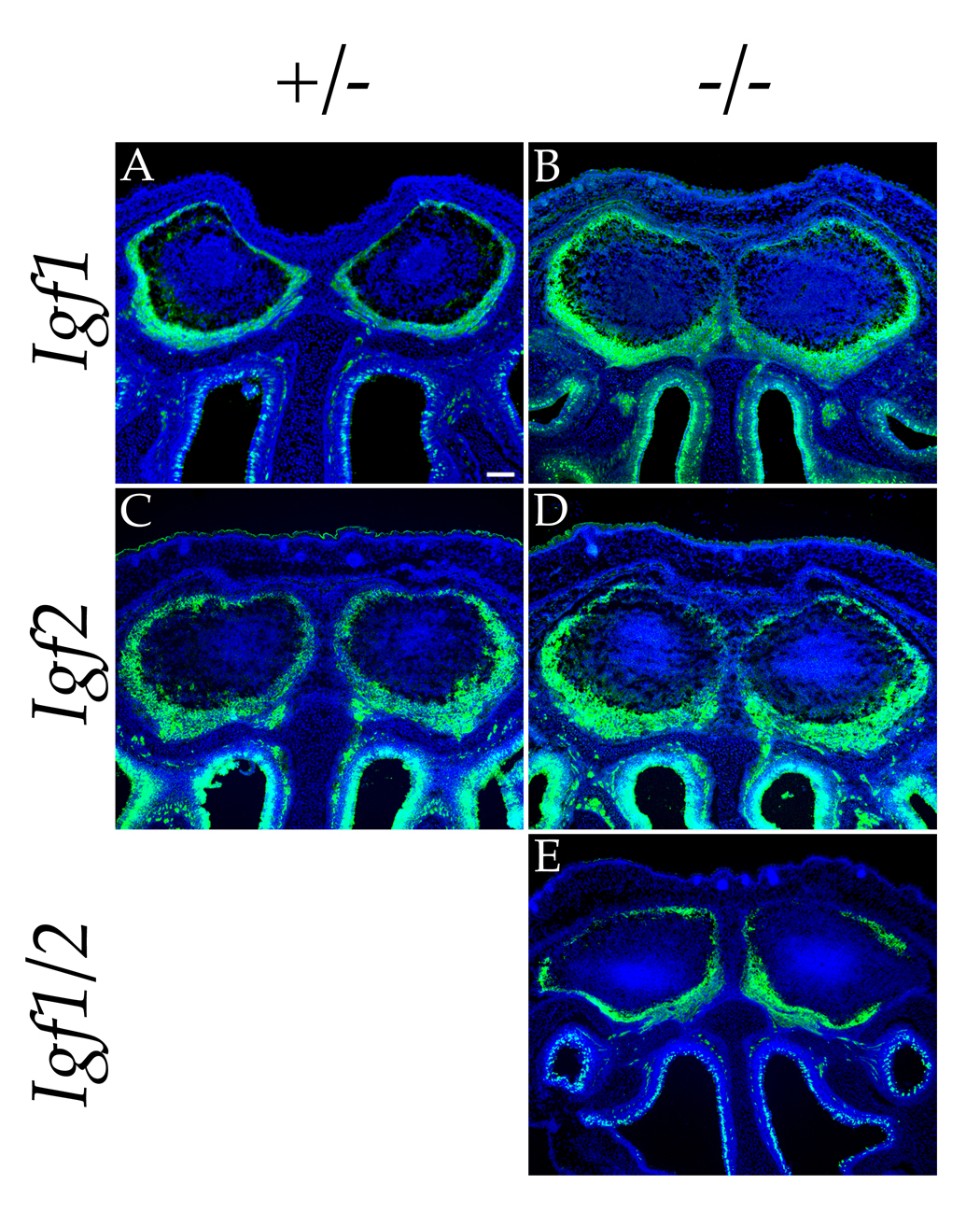
Immunolocalization of OMP expression (green) in coronal sections at E18.5 reveals that Igf1 and Igf2 single nullizygous mice do not have significant olfactory patterning defects (compare panel A with panel B and panel C with panel D). However, the elimination of both Igf1 and Igf2 in the Igf1/2 double null results in a severe decrease in innervation of the lateral olfactory bulbs, similar to the phenotype seen in the Igf1r nullizygote (compare panel E to Figure 2D). Cell nuclei were stained with Hoechst 33342 (blue). Scale bar = 100µm.
A straightforward and testable hypothesis explaining the difference between the Igf1r null phenotype and the lack of mutational consequences in the absence of either IGF ligand is that each of the ligands can compensate for the lack of the other. When we analyzed the innervation of olfactory bulbs in the Igf1−/−; Igf2−/− double mutant, we obtained results consistent with this hypothesis. As shown in Figure 3E, lateral innervation of the olfactory bulbs is dramatically decreased in the Igf1−/−;Igf2−/− double mutant, to an extent similar to that of the Igf1r−/− null phenotype (n = 2 mice for Igf1−/−; Igf2−/−). Thus, elimination of IGF signaling – either by genetically ablating both known IGF ligands or their common receptor – leads to the redistribution of olfactory sensory axons away from the lateral olfactory bulb toward the ventral-medial face.
Absence of IGF Signaling Causes Aberrant Targeting of Olfactory Sensory Neurons
Each olfactory bulb normally exhibits mirror symmetry, such that olfactory neurons expressing a given odorant receptor typically project their axons to two glomerular targets: one on the lateral face and one on the medial face of the olfactory bulb (Mombaerts et al., 1996; Nagao et al., 2000; Ressler et al., 1994; Vassar et al., 1994). The reduction of lateral innervation in the olfactory bulbs from both Igf1r and Igf1;Igf2 null mutants suggests that IGF signaling has a role in forming the mirror symmetry inherent in the olfactory sensory map. In one hypothesis, IGF signaling guides a subset of olfactory axons to the lateral bulb. Alternatively, IGF signaling does not function in the process of axon guidance, but rather provides trophic support with differential effects on subpopulations of olfactory neurons. In this latter scenario, neurons extending axons to the lateral olfactory bulb are more dependent on IGF signaling for their survival than medially-directed neurons. It has been shown, for example, that IGF signaling acts as a trophic factor for some neurons in vitro (Vincent et al., 2004) and in vivo (Chrysis et al., 2001; Ye et al., 1996). Thus, the loss of sensory axon innervation in the lateral portion of the olfactory bulb could be caused by the death of laterally-projecting neurons before or soon after their projections reach the bulb. We reasoned that if laterally-projecting cells die prematurely in the absence of IGF signaling, an olfactory neuron population expressing a single odorant receptor would form only the medial glomerulus instead of forming the usual pair of glomeruli.
To discriminate between these two hypotheses, we examined the projection pattern of olfactory neurons expressing the same odorant receptor, receptor P2. Axons projecting from cells expressing the P2 odorant receptor were visualized immunohistochemically using mice harboring an IRES-tau:lacZ insertion at the P2 locus (Mombaerts et al., 1996). Sections of olfactory bulbs from E18.5 mice wild type (data not shown) or heterozygous at the Igf1r locus were stained for β-galactosidase and showed a normal projection pattern of P2 olfactory neurons; axons formed glomeruli on both the medial and lateral faces of the olfactory bulb, a few hundred microns apart along the rostral-caudal axis (Figure 4A and B). On the other hand, in E18.5 Igf1r−/− littermates the axons from P2 neurons failed to extend into the lateral half of the bulb, and instead formed a glomerulus in the ventral-medial region (e.g., left bulb in Figure 4C). In some cases the position of the lateral glomerulus was shifted beyond the midline, into the medial half of the bulb (e.g., right bulb in Figure 4C). More caudal sections containing the medial P2 glomeruli revealed a slight shift in the position of these glomeruli in the Igf1r−/− bulb to more dorsal positions (Figure 4D), which was most likely due to the compression of the total sensory axon projection onto a smaller area of the olfactory bulb.
Figure 4. Misrouting of Odorant Receptor-Specific Olfactory Axons in the Absence of IGF Signaling.
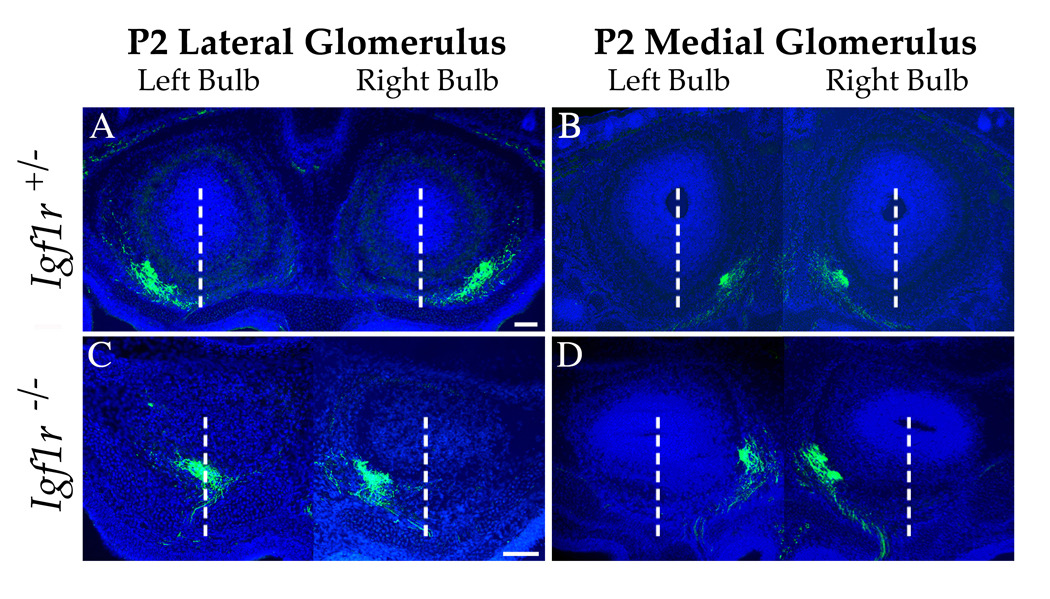
To localize the glomeruli of one class of odorant-specific olfactory axons, E18.5 P2-IRES-tau:lacZ mice either heterozygous (A and B) or nullizygous (C and D) at the Igf1r locus were stained with an anti-β-galactosidase antibody (green). (A, B) In Igf1r+/− mice, P2 neurons project to two glomeruli per olfactory bulb, one on the lateral (A) and, more caudally, one on the medial (B) face of the bulb. The position of these glomeruli is stereotyped between animals. (C, D) P2 neurons still form two glomeruli in the Igf1r nullizygous background. However, the lateral glomerulus (C) is shifted toward the medial hemisphere of the bulb, resulting in a distortion of the medial-lateral mirror symmetry of olfactory bulb innervation. Dashed white lines demarcate the midlines of each bulb. Note that in this particular individual, the lateral glomerulus in the right-hand bulb has shifted well beyond the midline. The medial glomeruli in the Igf1r nullizygote (D) appears to be in a position similar to that observed in the control (B), with only a small dorsomedial shift, which is likely due to the compression of the map into a smaller area of the bulb’s surface. In this example, the medial P2 axons form a doublet in the right bulb (D), a common phenomenon seen with this glomerulus (Royal and Key, 1999). Images of the left and right bulb are combined for clarity. Nuclei were stained with Hoechst 33342 (blue). Scale bar = 100µm.
Similar results were obtained from 7 P2-IRES-tau:lacZ; Igf1r−/− mice (vs. 5 littermate controls on the Igf1r+/− background). To quantitate this effect, we measured the positions of the P2 medial and P2 lateral glomeruli along the medial-lateral axis of the olfactory bulb, where in a given section containing a P2 glomerulus, zero corresponds to the medial margin of the mitral cell layer and 1.0 corresponds to the lateral margin of the mitral cell layer (Cutforth et al., 2003); a value of 0.5 approximates the midline of the olfactory bulb in each section. Using this metric, we found that the P2 medial glomerulus position was 0.08 +/− .08 (mean +/− standard error of the mean; n = 13 glomeruli), compared to −0.003 +/− 0.02 in the mutant (n = 6); this difference is not statistically significant (two-tailed t-test: p = 0.48). By contrast, the P2 “lateral” glomerulus on average was shifted beyond the bulb midline in the Igf1r−/− background (position = 0.38 +/− 0.09, n = 6), significantly different from the position measured in wild type animals (wild type position = 1.05 +/− 0.03, n = 6; p < 0.00004). These observations indicate that the olfactory axon patterning defect in Igf1r−/− mice is due to the misrouting of axons to ventromedial locations in the bulb, and is not due to the catastrophic loss of laterally projecting neurons.
IGF Can Serve as a Chemoattractant for Axon Growth Cones of Olfactory and Cerebellar Neurons
Our genetic evidence has revealed an unexpected role of IGF signaling in the patterning of sensory axon connections in the olfactory bulb. From these in vivo studies, however, it is unclear whether IGF acts as an instructive cue that directs the migration of olfactory axon growth cones, or alternatively functions indirectly to shape the olfactory sensory projection. To distinguish between these possibilities, we assessed the effects of IGF on axon growth cone migrations in cultured olfactory neurons. Olfactory neurons were isolated from neonatal rats and cultured for 24–48 hours. Cells were then transferred to serum-free medium, and actively migrating growth cones were identified and selected to test the action of IGF1 delivered from a pressure-injection pipette (Zheng et al., 1994). As shown in Figure 5A–C, growth cones were attracted to a gradient formed by a localized source of 200 µg/ml (26 µM) IGF1 (24° +/− 5.1° [mean +/− standard error of the mean], n = 12 growth cones scored). Under the conditions of this assay, the initial concentration of ligand at the growth cone is roughly 1/1000 the concentration in the pipette (Zheng et al., 1994), or 26 nM. This concentration corresponds to ~10x Kd of IGF1 for IGF1R (Jones and Clemmons, 1995); thus the observed effects occurred within the upper physiological range of IGF1/IGF1R action. The responses to IGF1 were significantly different from growth cone responses to a phosphate-buffered saline (PBS) control (−0.19° +/− 3.5°, n = 13; two tailed t-test: p < 0.001).
Figure 5. IGF1 is a Chemoattractant for Growth Cones of Olfactory Receptor Neurons In Vitro.
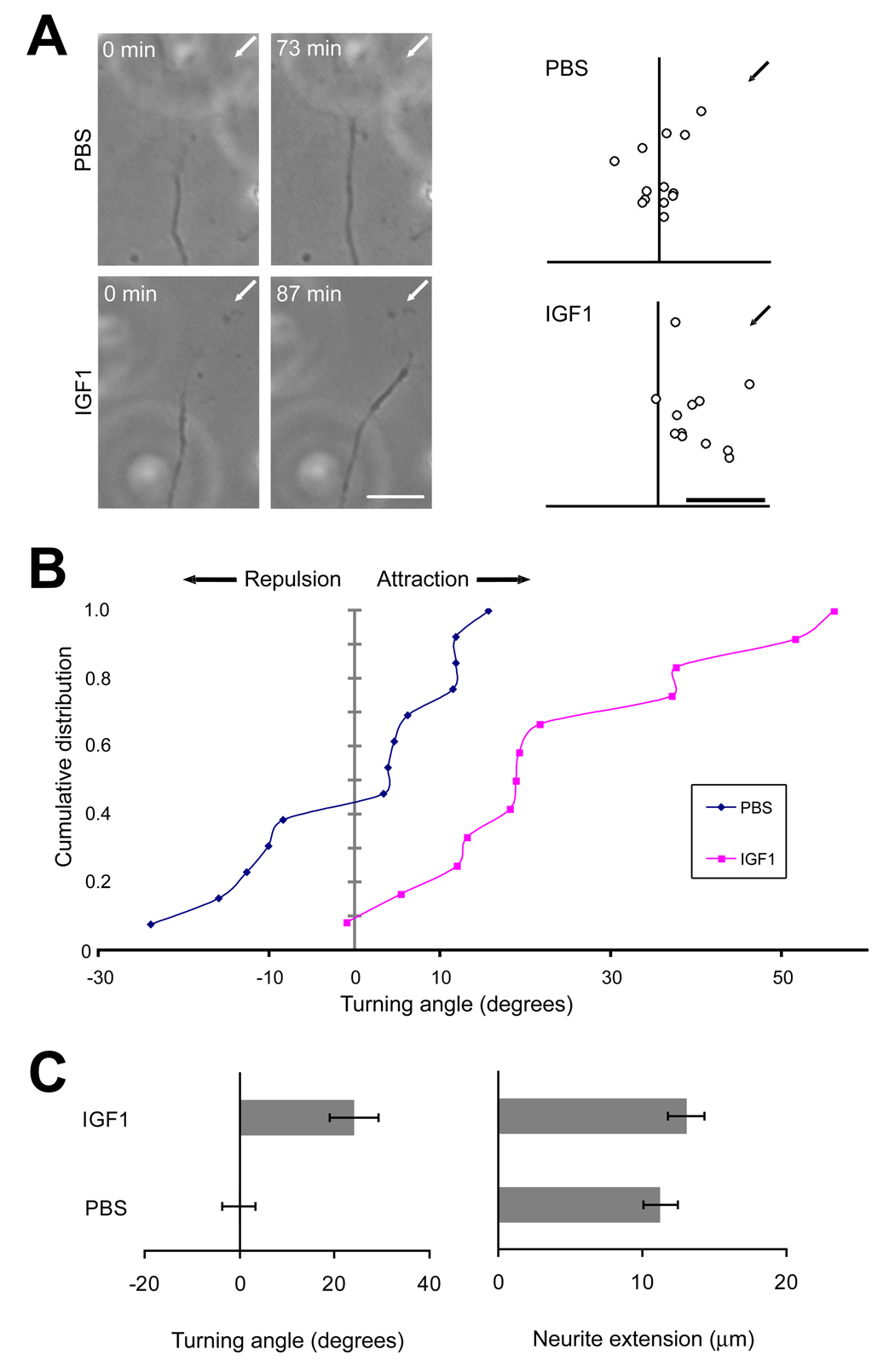
Growth cone turning assays were performed on cultured olfactory neurons isolated from neonatal rats. (A) Images of representative olfactory receptor neuron growth cones before and after exposure to a gradient of IGF1 (200 µg/ml) or PBS solution (left-hand panels). Arrows indicate the direction of the micropipette. Scattered points in the right-hand panel depict the final positions of growth cones. Scale bar = 10 µm. (B) Cumulative distribution of growth cone turning angles showing attraction of growth cones to IGF1. PBS control: n = 13 growth cones scored; IGF1: n = 12. (C) Mean turning angle (left) and neurite extension rate (right) in response to IGF1 and PBS. Responses under these two conditions were significantly different with regard to turning angle (two tailed t-test: p < 0.0001) but not neurite extension rate (p = 0.31).
It is possible that the observed attraction of olfactory neuron growth cones to IGF1 is due to increased rates of axon growth, and not the turning of growth cones per se. To address this possibility we calculated the net growth of the same axons used in the calculations of turning angles shown in Figure 5B and C. We found no differences in the growth of axons exposed to PBS or IGF1 (p = 0.31; Figure 5C). Thus, under these assay conditions IGF1 can elicit olfactory neuron growth cone attraction independent of any effect on axon growth rate. These results indicate that IGF functions as a chemoattractant to instruct the trajectory of growing olfactory axons.
To determine whether IGF can serve more generally as a chemoattractant cue for other neuronal cell types, we examined the effects of IGF1 on cultured cerebellar granule cells. Cerebellar granule neuron cultures are a well-established system for evaluating the activity of secreted proteins on axon guidance and for the elucidation of the signaling mechanisms underlying growth cone responses to such cues (Li et al., 2005; Xiang et al., 2002). Moreover, the relative ease of culturing cerebellar granule neurons (as compared to olfactory neurons) provides an opportunity to dissect the intracellular events downstream of IGF signaling. To perform the assays, granule neurons were isolated from rat cerebellum at postnatal (P) day 0 and placed in culture for 12–24 hours. Cells were then transferred to IGF-free medium for 30 minutes prior to exposure to a gradient generated by a point source of IGF1 (Zheng et al., 1994). Similar to our observations with cultured olfactory neurons, granule neuron growth cones were attracted to a gradient formed by a localized source of 20 µg/ml (2.6 µM) IGF1 (9.4° +/− 1.8°, n = 42 growth cones scored; Figure 6A–C). The turning of growth cones in response to IGF is dose-dependent, as elevating the concentration of IGF1 to 200 µg/ml in the source pipette increased the turning angle to 13.3° +/− 4.4° (n=35; Figure 6A–C). These responses were significantly different from growth cone responses to PBS (−1.3° +/− 1.7°, n = 31; two tailed t-test: p < 0.0001 for 20 µg/ml IGF1 vs. PBS and p < 0.01 for 200 µg/ml IGF1 vs. PBS).
Figure 6. IGF1 is a Chemoattractant for Cerebellar Granule Neuron Growth Cones In Vitro.
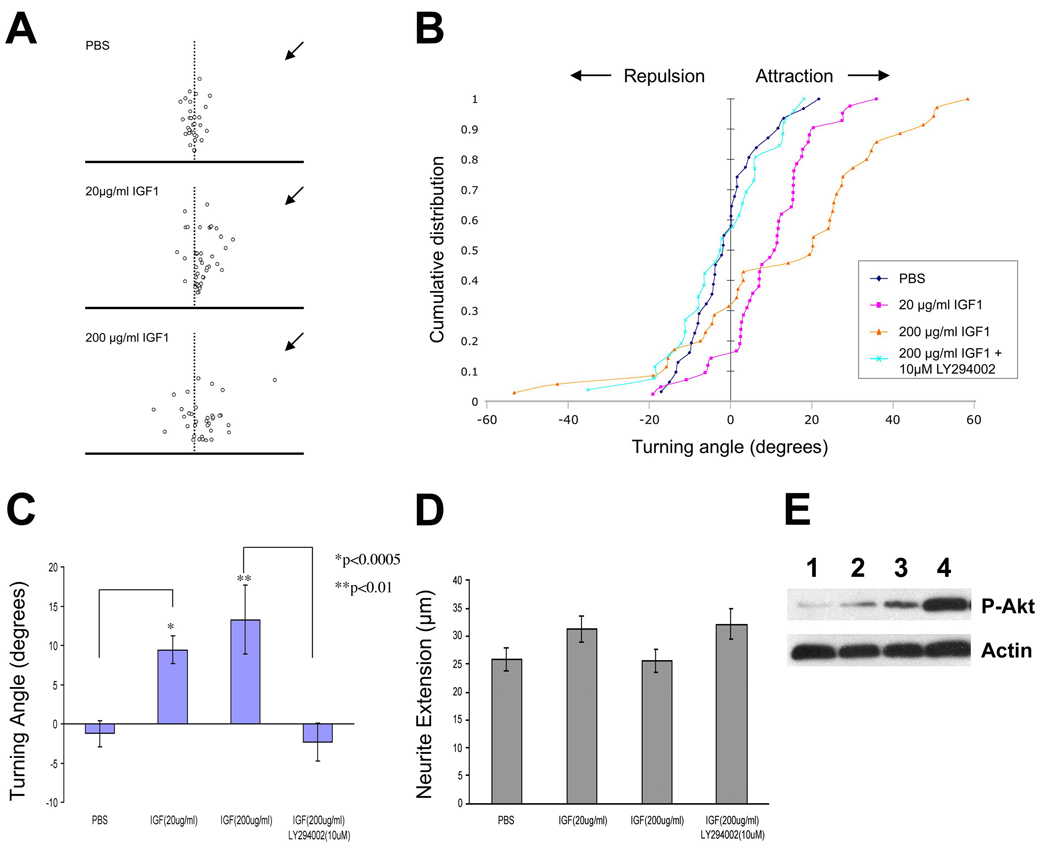
Growth cone turning assays were performed on cultured rat cerebellar granule neurons. Actively migrating growth cones were exposed to a gradient of IGF1 from a point source set at an initial 45 degree angle. (A) Scatter plots showing the relative angle (X-axis) and total neurite extension (Y-axis) of individual growth cones following exposure to PBS, 20µg/ml IGF1, or 200 µg/ml IGF1, as indicated. For each plot, the origin corresponds to the center of the growth cone at the beginning of the experiment, and the direction of the gradient is indicated with an arrow. (B) Cumulative distribution of turning angles showing that growth cones are attracted to a gradient of IGF1 in a dose dependent manner compared to PBS control, and that this attraction is abolished in the presence of the PI3 kinase inhibitor, LY294002. PBS control: n = 31 growth cones assayed; 20µg/ml IGF1: n = 42; 200 µg/ml IGF1: n = 35; 200 µg/ml IGF1 + 10µM LY294002: n = 26. (C) Quantitation of the data shown in panel A demonstrates that the attraction of growth cones to IGF1 is statistically significantly different as compared to control (PBS; see text for details). Inhibition of PI3 kinase with LY294002 results in a significant reduction in IGF1-mediated attraction. (D) Measurement of net extension of the same growth cones over a 30 minute period shows no effect of IGF1 on neurite outgrowth under these assay conditions. (E) Western blotting for phospho-Akt, a downstream target of IGF1R/PI3 kinase, reveals a dose-dependent accumulation of phosphorylated Akt in response to IGF1 in cerebellar granule cells (top panel). An anti-actin blot from the same gel serves as a loading control (bottom panel). Lane 1, no IGF1; lane 2, 2 ng/ml IGF1; lane 3, 20 ng/ml IGF1; lane 4, 200 ng/ml IGF1.
PI3 kinase is a downstream target of IGF1R (Yamamoto et al., 1992) which, among other actions, appears to mediate the activity of multiple axon guidance cues (Ming et al., 1999; Song and Poo, 1999). We therefore asked whether the observed attraction of granule neuron growth cones to IGF1 is PI3 kinase-dependent, using two different approaches. First, we determined whether Akt, a direct downstream target of PI3 kinase (Yamamoto et al., 1992), is phosphorylated in response to IGF1. Serum-starved cultures of cerebellar granule neurons were treated with different concentrations of IGF1 (2, 20, or 200 ng/ml) for 30 minutes, and the presence of phosphorylated Akt was evaluated by Western blotting using an anti-phospho-Akt antibody. As shown in Figure 6E, IGF1 elicited a dose-dependent increase in phospho-Akt, indicating that PI3 kinase is indeed activated by IGF1 in these cells. In a second experiment, we measured growth cone turning responses to IGF1 in the presence of LY294002, a specific PI3 kinase inhibitor. We observed that growth cones did not show any attraction to 200 µg/ml IGF1 with 10 µM LY294002 present in the culture medium (Figure 6B and C; turning angle = −2.3° +/− 2.4°, n=26; p > 0.7 compared to PBS control), demonstrating that the attraction of growth cones to IGF1 is dependent on PI3 kinase signaling. Finally, we found no differences in the growth of axons exposed to PBS, low or high levels of IGF1, or IGF1 in the presence of LY294002 (Figure 6D). Thus, under these assay conditions IGF1 can elicit growth cone attraction independent of any effect on axon growth rate. Together these results indicate that IGF can serve as a chemoattractant cue for growth cones of both olfactory neurons and cerebellar granule neurons, suggesting a general role of IGF signaling in axon guidance.
Discussion
IGF Signaling Guides the Formation of the Olfactory Sensory Map
In the present study, we identified IGF signaling as an important determinant in the development of the olfactory sensory map. Specifically, IGF signaling is required for the correct positioning of glomeruli in the lateral olfactory bulb. In IGF mutants, glomeruli normally positioned in the lateral half of the bulb are shifted to ventromedial locations. The abnormal accumulation of fibers in the ventromedial bulb suggests a rotation and compression of the sensory map from dorsal-lateral positions toward the ventral midline. We interpret these observations to suggest that IGF-mediated signaling in the ingrowing olfactory axons is required for their proper innervation in the olfactory bulb. This view should be tempered by the possibility that the olfactory phenotype of IGF mutants is due instead to indirect effects that lead secondarily to axonal misrouting. For example, loss of IGF signaling could cause a change in gene expression within the olfactory neurons, causing them to respond differently to target-derived axon guidance cues. Alternatively, the misplacement or absence of cells in specific bulb regions could result in the absence of innervation where such cells are missing. It should be noted, however, that the convergence of olfactory sensory axons to both medial and lateral sites can occur in mouse mutants in which olfactory bulb neurons are either missing or disorganized (Bulfone et al., 1998; Royal et al., 2002; St John et al., 2003). Moreover, we found that IGF1 can serve as a chemoattractant for growth cones of cultured olfactory neurons, consistent with the notion that IGF is acting directly on olfactory axons in vivo.
IGF Signaling and Axon Guidance
It is generally thought that the IGFs function predominantly as growth-promoting factors (Efstratiadis, 1998). Although a loss of cells in specific brain nuclei and axons of certain nerve tracts has been observed in an Igf1−/− mutant (Beck et al., 1995), there has been no clear evidence to suggest effects on neuronal patterning that are not secondary to perturbations in cell proliferation or cell survival. Nonetheless, a recent study has demonstrated that the axons forming the corticospinal tract are dependent on IGF signaling for growth; IGF1 applied to neurons in culture stimulated the rate of axon extension, and inhibition of IGF1R with a function-blocking antibody in vivo perturbed axon outgrowth (Ozdinler and Macklis, 2006). Here we extend these findings by showing an unexpected role for IGF signaling in axon guidance. In the olfactory system, disruption of IGF signaling causes a dramatic misrouting of axons normally destined to innervate the lateral olfactory bulb. We further show that IGF functions as a chemoattractant for growth cones of cultured olfactory neurons and cerebellar granule neurons, independent of any effect IGF may have on promoting axon outgrowth. We hypothesize that IGFs have the potential to serve more broadly as instructive cues that guide the migrations of growing axons in the developing nervous system. Indeed, PI3 kinase, a downstream effector of IGF signaling through IGF1R (Yamamoto et al., 1992), has been shown to mediate growth cone turning in response to a variety of axon guidance cues (Song and Poo, 1999).
From our present studies it is not entirely clear whether olfactory axons projecting to the lateral olfactory bulb depend on IGF signaling for axon outgrowth, as in the corticospinal tract (Ozdinler and Macklis, 2006). However, we never observe axons stalled outside of the olfactory bulb in IGF mutants (e.g., see Figure 2), and a close examination of P2 axon trajectories indicates that axon outgrowth is not inhibited in IGF mutants. In wild type mice, P2 axons entering the bulb through openings in the lateral cribriform plate turn laterally and extend toward their target glomerulus (Figure 7C). In contrast, P2 axons crossing the lateral cribriform plate appear to turn medially upon entering the bulb in the Igf1r−/− mutant, and extend across the bulb’s ventral surface to form a misplaced glomerulus (Figure 7D). In both cases the labeled axons traverse a comparable distance after entering the olfactory nerve layer, suggesting that IGF signaling is not strictly required for the outgrowth of olfactory axons.
Figure 7. Model of IGF Activity in the Formation of the Olfactory Sensory Map.
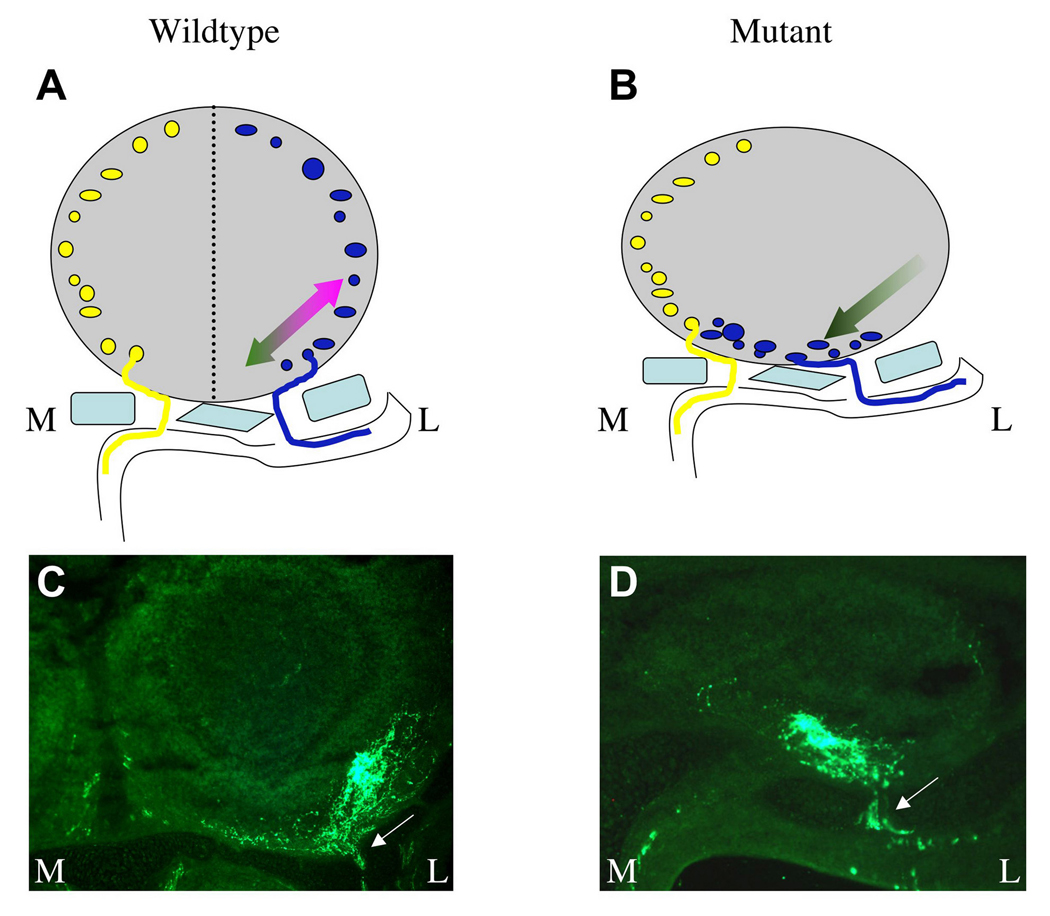
(A) Under normal conditions, medially- (gold) and laterally- (blue) disposed olfactory axons cross the cribriform plate (light blue) and extend to medial and lateral glomeruli, respectively, forming a mirror symmetry in the olfactory bulb (dotted line represents the plane of symmetry, which extends out of the plane of the figure). We hypothesize that the position of a lateral glomerulus is determined through a “push-pull” mechanism. In this model, IGF ligands expressed in the olfactory bulb attract axons exiting the lateral cribriform plate to grow dorsolaterally (magenta arrow). This orsolateral attraction is opposed by an unknown cue(s) that influences the axons to grow ventromedially (dark green arrow). Together these opposing forces play a role in determining the positions of glomeruli in the lateral olfactory bulb. (B) In IGF mutants, axons no longer respond to IGF (Igf1r−/−) or IGFs are missing (Igf1−/−;Igf2−/−), leaving the medial “push” (dark green arrow) unopposed by the IGF-dependent lateral “pull.” This results in a severe distortion of the mirror symmetry within the olfactory bulb, with a lateral → ventromedial rotation and compression of the sensory map. Axons that normally extend to the medial olfactory bulb are largely unaffected by the absence of IGF signaling, and are probably responding to another attractive cue(s) to enter the medial bulb. (C, D) Examples of tau:lacZ-labeled P2 axons from the lateral olfactory epithelium as they penetrate and cross the cribriform plate (arrows) and extend to their target glomeruli. In the wild type background, axons turn and extend laterally upon exiting the cribriform plate (C). In the Igf1r−/− mutant, axons instead turn medially and extend a comparable distance to an ectopic site in the ventromedial bulb (D). Thus the observed distortion of the olfactory sensory map in IGF mutants is likely the result of the misrouting of axons normally destined to innervated the lateral olfactory bulb.
Mechanisms of Olfactory Sensory Map Formation
The stereotypic nature of the olfactory sensory map suggests that the precise convergence and targeting of sensory axons in the olfactory bulb is the result of a response of specific sensory axons to spatially restricted guidance cues in the target tissue and along the axonal trajectory. In one model, a hierarchy of such guidance cues directs axons of neurons expressing the same odorant receptor to their common glomerular targets in the olfactory bulb (Lin and Ngai, 1999; St John et al., 2002). For example, neurons expressing a specific odorant receptor are segregated within defined zones in the epithelium along the dorsomedial-ventrolateral axis (Miyamichi et al., 2005; Ressler et al., 1993; Vassar et al., 1993). While axons arising from each zone project to a corresponding dorsal-ventral zone in the olfactory bulb (Miyamichi et al., 2005; Mori et al., 1999), within these latter zones odorant receptor-specific axons converge to form discrete glomeruli. Zone-to-zone projection may be established by a mechanism that restricts the olfactory axons to broad domains in the bulb (Cho et al., 2007), while additional levels of control direct axons to converge to their appropriate glomeruli within these domains. Interestingly, the odorant receptor itself – via cAMP signaling – influences glomerular position along the anterior-posterior axis of the olfactory bulb (Imai et al., 2006; Mombaerts et al., 1996; Wang et al., 1998), although it is not the sole determinant of the final position of the glomerulus. Thus, zone-to-zone projections restrict axons along the dorsal-ventral axis of the olfactory bulb and odorant receptors influence glomerular positioning along the anterior-posterior axis. Here we show that IGF signaling shapes the olfactory axon projection along the medial-lateral axis, the bulb’s third principal axis. The sorting of axons to the medial and lateral hemispheres of the olfactory bulb represents a key developmental choice necessary for the establishment of mirror symmetry in the olfactory sensory map.
What mechanisms are used to influence this choice? Axons innervating the medial or lateral olfactory bulb originate from neurons found in medial or lateral positions of the olfactory epithelium, respectively (Levai et al., 2003). In a simple scenario, axons would merely need to grow “up” into the bulb in order to innervate the medial and lateral hemispheres. Consistent with this idea, the chemorepellant Sema3A is expressed in the medial bulb and has been suggested to provide a midline barrier for olfactory axons expressing neuropilin-1, the Sema3A receptor (Schwarting et al., 2000; Taniguchi et al., 2003). However, a null mutation in the Sema3A gene results in only a slight anterior-medial shift of the two mirror-symmetric maps (Taniguchi et al., 2003). The lateral > medial gradient of IGF1 in the rostral olfactory bulb at E14.5 (Figure 1) suggests that the differential localization of IGF1 may attract some olfactory axons preferentially to the lateral bulb. However, this gradient is subtle, is restricted to the rostral olfactory bulb, and disappears by E15.5 (data not shown). It is also possible that any one of the numerous IGF binding proteins encoded in the genome (Efstratiadis, 1998) may sculpt the spatial distribution of available IGF ligands along the olfactory projection. Yet a null mutation in the Igf1 gene does not cause a demonstrable perturbation in the olfactory sensory projection (Figure 3), suggesting that the mechanisms influencing medial vs. lateral projection decisions likely involve a complex interplay between multiple factors.
While it is formally possible that the response of olfactory neurons to other axon guidance cues is itself dependent on IGF signaling, we favor a model in which olfactory axons are guided by IGFs (and possibly other factors) to their final glomerular position. (Figure 7). In this model, all olfactory axons, regardless of their origin, are attracted to the medial bulb. Axons originating from the lateral olfactory epithelium therefore require a counterbalancing lateral attraction in order to extend into the lateral hemisphere of the olfactory bulb. We propose that IGF ligands serve as this attractive cue, with the ultimate location of a given glomerulus in the lateral hemisphere a function of the medial “push” and lateral “pull” imposed on the sensory axon growth cones (Figure 7A). In the absence of IGF signaling – as in the IGF mutants studied here – the lateral “pull” is severely reduced and the medial “push” (which may arise from an attractive medial cue or repulsive lateral cue) is revealed, resulting in the misrouting of axons to ectopic locations in the ventral bulb (Figure 7B). Although our model invokes the activity of a hypothetical factor to provide a medial “push” to the axons, an analogous “push-pull” mechanism has been described in the development of the retinotopic map, where countergradients specify the location of retinal ganglion cell axon terminals in the target tissue (Schmitt et al., 2006).
Why do the lateral axons not target to the same location as the medial glomerulus in IGF mutants? Without the appropriate attractive cue to turn laterally, the lateral axons find themselves in a novel environment. We speculate that they form glomeruli in positions based upon the molecular cues present in their new target environment, which may be very different from that found in the lateral hemisphere. It is interesting to note that the position of the medial glomerulus is largely unaffected in Igf1r−/− mice; axons innervating this glomerulus appear to follow their normal targeting strategy independent of IGF signaling. The behavior of these axons may reflect the presence of other attractive cues in the medial bulb that compensate for the absence of IGF signaling in the mutant background.
It remains unclear at present what factors or cues specify the exact location of individual glomeruli. Nonetheless, the data presented here clearly identify a role of IGF signaling in the formation of the olfactory sensory map and add the IGFs to a growing list of molecules involved in the patterning of neuronal connections in the developing nervous system.
Experimental Procedures
Mouse Strains
All mouse strains used in this study were on a mixed 129/Bl6 background. Igf1 and Igf2 mutants are described in (DeChiara et al., 1990; Liu et al., 1993). Igf1r mutants, described in (Liu et al., 1993), were crossed with OMP-IRES-tau:lacZ and P2-IRES-tau:lacZ mice described in (Mombaerts et al., 1996).
Immunohistochemistry
Whole heads from different embryonic stages were fixed for 2 hr at room temperature in 4% paraformaldehyde, followed by an overnight incubation in 30% sucrose, 1X PBS at 4°C. Tissue was then mounted in TBS tissue freezing medium (Triangle Biosciences) and frozen at −80°C until sectioned. Cryosections were taken at 12 µm thickness unless indicated otherwise.
For immunohistochemistry, slides were incubated in PBST (1XPBS, 0.1% Triton X-100) for five minutes followed by a one hour incubation in blocking solution (1mM Tris pH7.5, 10% heat inactivated normal goat serum [HINGS], 0.1% Triton X-100) before primary antibody was added. Rabbit anti-β-galactosidase (1:900, Cappel) or rabbit anti-GFP (purified IgG; 1:900, Invitrogen) were incubated for 1 hr at room temperature followed by incubation at 4°C overnight. For IGF1R staining, sections were incubated in 3% H2O2 for 45 min prior to incubation in blocking solution; chicken anti-IGF1R (1:10, Upstate) was incubated for 2 hr at room temperature followed by incubation at 4°C overnight and visualized using an HRP-conjugated goat anti-chicken antibody (1:200, AbCam) and Cy3 tyramide (Perkin Elmer). IGF1R was also localized using a rabbit anti-IGF1R antibody (1:50, Cell Signaling Technology) following treatment of tissue sections in boiling sodium citrate buffer. Goat anti-IGF1 and goat anti-IGF2 (R&D systems) were each used at 1:20 dilution. Goat anti-OMP (Wako) was used at 1:1000. For goat primary antibodies, donkey serum replaced goat serum in the blocking solution. Signal was detected using Alexa 488-, or Alexa 568- or Alexa594-conjugated secondary antibodies (Invitrogen). Sections were counterstained with Hoechst 33342 to visualize the positions of nuclei. RNA in situ hybridizations were performed as described previously (Speca et al., 1999).
Neuronal Cell Culture and Growth Cone Turning Assays
Olfactory septa and turbinates were collected from P0-P2 neonatal rats and incubated in papain (20 units/ml in PBS) at 37°C for 30 min. The solution was then removed and replaced with 10% fetal bovine serum (FBS) in Neurobasal medium, after which the remaining bony pieces and large aggregates were triturated gently with a glass pipette. The dissociated cells were pelleted by centrifugation, resuspended in prewarmed olfactory neuron culture media (Neurobasal plus 2% B27, 10 µg/ml Insulin, 2 mM Glutamax, 10 ng/ml BDNF, 25 ng/ml NGF) and plated on laminin-coated coverslips. After culturing for 24–48hr at 37°C, coverslips were transferred to a heated chamber on the stage of a microscope and incubated in serum-free L15 medium for at least 30 min at ~ 37°C before the growth cone turning assay. Cerebellar granule neurons from P0 rats were dissociated and plated at low density (105/dish) onto laminin-coated coverslips (Xiang et al., 2002). After culturing for 12–24 hr at 37°C, coverslips were transferred to a heated chamber on the stage of a microscope and incubated in serum-free L15 medium for at least 30 min at ~ 37°C.
Microscopic gradients of IGF were produced as described previously (Zheng et al., 1994) except that the tip of the pipette was located 75 µm away from the growth cone center. Images of neurites were recorded and analyzed using Scion Image 4.0.2. Turning angle was defined as the angle between the original direction of neurite’s extension and a straight line connecting the growth cone position at the start and the end of the assay period. Procedures for both cell types were identical, except that the final positions of the olfactory neuron growth cones were measured following 60 to 90 minutes exposure to the gradient (vs. 1 hr for cerebellar granule neurons), and only neurites that extended > 5 µm during the 60~90 min period were included in the analysis for olfactory neurons. For pharmacological treatment of cerebellar granule neurons, LY294002 was applied 30 min before the turning assay and maintained throughout the duration of the assay.
Western Blotting
Cerebellar granule cells were cultured at high density (2×105/dish) in 35 mm tissue culture dishes and serum starved for 2 hr before stimulation with IGF1. Cells were harvested in lysis buffer (0.1% SDS, 1% Nonidet P-40, 1% glycerin, 50 mM HEPES, pH7.4, 2 mM EDTA and 100 mM NaCl; 200 µl/dish). Proteins were separated by electrophoresis on 10% SDS–polyacrylamide gels and transferred onto PVDF membranes. Blots were blocked for 3hr at room temperature in 5% BSA, incubated with a polyclonal antibody specific for phospho-Akt (1:1000, R&D Systems) overnight at 4°C, rinsed and incubated for 1 hr at room temperature with a horseradish-peroxidase-conjugated goat anti-rabbit IgG (1:10,000; Biorad). Chemiluminescence detection was performed with an ECL kit (Pierce).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute on Deafness and Other Communications Disorders (J.N.), the Natural Science Foundation of China (X.-b.Y., 30625023), and from the National Cancer Institute (A.E., 1P01 CA97403, Project 2). We thank Richard Axel for generously providing mouse strains and Mu-ming Poo for insightful discussions throughout the course of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–730. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Wang F, Hevner R, Anderson S, Cutforth T, Chen S, Meneses J, Pedersen R, Axel R, Rubenstein JLR. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron. 1998;21:1273–1282. doi: 10.1016/s0896-6273(00)80647-9. [DOI] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, Axel R. Allelic Inactivation Regulates Olfactory Receptor Gene Expression. Cell. 1994;78:823–834. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- Cho JH, Lepine M, Andrews W, Parnavelas J, Cloutier JF. Requirement for Slit-1 and Robo-2 in zonal segregation of olfactory sensory neuron axons in the main olfactory bulb. J Neurosci. 2007;27:9094–9104. doi: 10.1523/JNEUROSCI.2217-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysis D, Calikoglu AS, Ye P, D'Ercole AJ. Insulin-like growth factor-I overexpression attenuates cerebellar apoptosis by altering the expression of Bcl family proteins in a developmentally specific manner. J Neurosci. 2001;21:1481–1489. doi: 10.1523/JNEUROSCI.21-05-01481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutforth T, Moring L, Mendelsohn M, Nemes A, Shah NM, Kim MM, Frisen J, Axel R. Axonal ephrin-As and odorant receptors: coordinate determination of the olfactory sensory map. Cell. 2003;114:311–322. doi: 10.1016/s0092-8674(03)00568-3. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A. Genetics of mouse growth. Int J Dev Biol. 1998;42:955–976. [PubMed] [Google Scholar]
- Feinstein P, Mombaerts P. A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell. 2004;117:817–831. doi: 10.1016/j.cell.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ferrari CC, Johnson BA, Leon M, Pixley SK. Spatiotemporal distribution of the insulin-like growth factor receptor in the rat olfactory bulb. Neurochem Res. 2003;28:29–43. doi: 10.1023/a:1021639926941. [DOI] [PubMed] [Google Scholar]
- Imai T, Sakano H. Roles of odorant receptors in projecting axons in the mouse olfactory system. Curr Opin Neurobiol. 2007;17:507–515. doi: 10.1016/j.conb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Imai T, Suzuki M, Sakano H. Odorant receptor-derived cAMP signals direct axonal targeting. Science. 2006;314:657–661. doi: 10.1126/science.1131794. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Rhoades RW, Bennett-Clarke CA. The formation of a cortical somatotopic map. Trends Neurosci. 1995;18:402–407. doi: 10.1016/0166-2236(95)93937-s. [DOI] [PubMed] [Google Scholar]
- Laurino L, Wang XX, de la Houssaye BA, Sosa L, Dupraz S, Caceres A, Pfenninger KH, Quiroga S. PI3K activation by IGF-1 is essential for the regulation of membrane expansion at the nerve growth cone. J Cell Sci. 2005;118:3653–3662. doi: 10.1242/jcs.02490. [DOI] [PubMed] [Google Scholar]
- Levai O, Breer H, Strotmann J. Subzonal organization of olfactory sensory neurons projecting to distinct glomeruli within the mouse olfactory bulb. J Comp Neurol. 2003;458:209–220. doi: 10.1002/cne.10559. [DOI] [PubMed] [Google Scholar]
- Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- Lin DM, Ngai J. Development of the vertebrate main olfactory system. Current Opinion in Neurobiology. 1999;9:74–78. doi: 10.1016/s0959-4388(99)80009-9. [DOI] [PubMed] [Google Scholar]
- Lin DM, Yang YH, Scolnick JA, Brunet LJ, Marsh H, Peng V, Okazaki Y, Hayashizaki Y, Speed TP, Ngai J. Spatial patterns of gene expression in the olfactory bulb. Proc Natl Acad Sci U S A. 2004;101:12718–12723. doi: 10.1073/pnas.0404872101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- McLaughlin T, O'Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- Ming G, Song H, Berninger B, Inagaki N, Tessier-Lavigne M, Poo M. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Miyamichi K, Serizawa S, Kimura HM, Sakano H. Continuous and overlapping expression domains of odorant receptor genes in the olfactory epithelium determine the dorsal/ventral positioning of glomeruli in the olfactory bulb. J Neurosci. 2005;25:3586–3592. doi: 10.1523/JNEUROSCI.0324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol. 2006;22:713–737. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Schachner M, Montag D. Misguided axonal projections, neural cell adhesion molecule 180 mRNA upregulation, and altered behavior in mice deficient for the close homolog of L1. Mol Cell Biol. 2002;22:7967–7981. doi: 10.1128/MCB.22.22.7967-7981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Nagao H, Yoshihara Y, Mitsui S, Fujisawa H, Mori K. Two mirror-image sensory maps with domain organization in the mouse main olfactory bulb. Neuroreport. 2000;11:3023–3027. doi: 10.1097/00001756-200009110-00039. [DOI] [PubMed] [Google Scholar]
- Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- Puche AC, Poirier F, Hair M, Bartlett PF, Key B. Role of galectin-1 in the developing mouse olfactory system. Develop Biol. 1996;179:274–287. doi: 10.1006/dbio.1996.0257. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A Zonal Organization of Odorant Receptor Gene Expression in the Olfactory Epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information Coding in the Olfactory System - Evidence for a Stereotyped and Highly Organized Epitope Map in the Olfactory Bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Royal SJ, Gambello MJ, Wynshaw-Boris A, Key B, Clarris HJ. Laminar disorganisation of mitral cells in the olfactory bulb does not affect topographic targeting of primary olfactory axons. Brain Res. 2002;932:1–9. doi: 10.1016/s0006-8993(01)03384-4. [DOI] [PubMed] [Google Scholar]
- Royal SJ, Key B. Development of P2 olfactory glomeruli in P2-internal ribosome entry site-tau-lacZ transgenic mice. Journal of Neuroscience. 1999;19:9856–9864. doi: 10.1523/JNEUROSCI.19-22-09856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Schwarting GA, Kostek C, Ahmad N, Dibble C, Pays L, Puschel AW. Semaphorin 3A is required for guidance of olfactory axons in mice. Journal of Neuroscience. 2000;20:7691–7697. doi: 10.1523/JNEUROSCI.20-20-07691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Takeuchi H, Yamagishi Y, Suzuki M, Sakano H. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 2006;127:1057–1069. doi: 10.1016/j.cell.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- Sosa L, Dupraz S, Laurino L, Bollati F, Bisbal M, Caceres A, Pfenninger KH, Quiroga S. IGF-1 receptor is essential for the establishment of hippocampal neuronal polarity. Nat Neurosci. 2006;9:993–995. doi: 10.1038/nn1742. [DOI] [PubMed] [Google Scholar]
- Speca DJ, Lin DM, Sorensen PW, Isacoff EY, Ngai J, Dittman AH. Functional identification of a goldfish odorant receptor. Neuron. 1999;23:487–498. doi: 10.1016/s0896-6273(00)80802-8. [DOI] [PubMed] [Google Scholar]
- St John JA, Clarris HJ, Key B. Multiple axon guidance cues establish the olfactory topographic map: how do these cues interact? Int J Dev Biol. 2002;46:639–647. [PubMed] [Google Scholar]
- St John JA, Clarris HJ, McKeown S, Royal S, Key B. Sorting and convergence of primary olfactory axons are independent of the olfactory bulb. J Comp Neurol. 2003;464:131–140. doi: 10.1002/cne.10777. [DOI] [PubMed] [Google Scholar]
- Stylianopoulou F, Herbert J, Soares MB, Efstratiadis A. Expression of the insulin-like growth factor II gene in the choroid plexus and the leptomeninges of the adult rat central nervous system. Proc Natl Acad Sci U S A. 1988;85:141–145. doi: 10.1073/pnas.85.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Takeda M. Expression of insulin-like growth factor family in the rat olfactory epithelium. Anat Embryol (Berl) 2002;205:401–405. doi: 10.1007/s00429-002-0266-5. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Nagao H, Takahashi YK, Yamaguchi M, Mitsui S, Yagi T, Mori K, Shimizu T. Distorted odor maps in the olfactory bulb of semaphorin 3A-deficient mice. J Neurosci. 2003;23:1390–1397. doi: 10.1523/JNEUROSCI.23-04-01390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisay KT, Bartlett PF, Key B. Primary olfactory axons form ectopic glomeruli in mice lacking p75NTR. J Comp Neurol. 2000;428:656–670. doi: 10.1002/1096-9861(20001225)428:4<656::aid-cne6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Treloar H, Tomasiewicz H, Magnuson T, Key B. The central pathway of primary olfactory axons is abnormal in mice lacking the N-CAM-180 isoform. J Neurobiol. 1997;32:643–658. doi: 10.1002/(sici)1097-4695(19970620)32:7<643::aid-neu1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial Segregation of Odorant Receptor Expression in the Mammalian Olfactory Epithelium. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Feldman EL, Song DK, Jung V, Schild A, Zhang W, Imperiale MJ, Boulis NM. Adeno-associated viral-mediated insulin-like growth factor delivery protects motor neurons in vitro. Neuromolecular Med. 2004;6:79–85. doi: 10.1385/NMM:6:2-3:079. [DOI] [PubMed] [Google Scholar]
- Walz A, Mombaerts P, Greer CA, Treloar HB. Disrupted compartmental organization of axons and dendrites within olfactory glomeruli of mice deficient in the olfactory cell adhesion molecule, OCAM. Mol Cell Neurosci. 2006;32:1–14. doi: 10.1016/j.mcn.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Walz A, Rodriguez I, Mombaerts P. Aberrant sensory innervation of the olfactory bulb in neuropilin-2 mutant mice. J Neurosci. 2002;22:4025–4035. doi: 10.1523/JNEUROSCI.22-10-04025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Nemes A, Mendelsohn M, Axel R. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93:47–60. doi: 10.1016/s0092-8674(00)81145-9. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Li Y, Zhang Z, Cui K, Wang S, Yuan XB, Wu CP, Poo MM, Duan S. Nerve growth cone guidance mediated by G protein-coupled receptors. Nat Neurosci. 2002;5:843–848. doi: 10.1038/nn899. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Lapetina EG, Moxham CP. Insulin like growth factor-I induces limited association of phosphatidylinositol 3-kinase to its receptor. Endocrinology. 1992;130:1490–1498. doi: 10.1210/endo.130.3.1311242. [DOI] [PubMed] [Google Scholar]
- Ye P, Xing Y, Dai Z, D'Ercole AJ. In vivo actions of insulin-like growth factor-I (IGF-I) on cerebellum development in transgenic mice: evidence that IGF-I increases proliferation of granule cell progenitors. Develop Brain Res. 1996;95:44–54. doi: 10.1016/0165-3806(96)00492-0. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


