Abstract
Activation of β-adrenergic receptors (β-ARs) enhances hippocampal memory consolidation and long-term potentiation (LTP), a likely mechanism for memory storage. One signaling pathway linked to β-AR activation is the cAMP-PKA pathway. PKA is critical for the consolidation of hippocampal long-term memory and for the expression of some forms of long-lasting hippocampal LTP. How does β-AR activation affect the PKA-dependence, and persistence, of LTP elicited by distinct stimulation frequencies? Here, we use in vitro electrophysiology to show that patterns of stimulation determine the temporal phase of LTP affected by β-AR activation. In addition, only specific patterns of stimulation recruit PKA-dependent LTP following β-AR activation. Impairments of PKA-dependent LTP maintenance generated by pharmacologic or genetic deficiency of PKA activity are also abolished by concurrent activation of β-ARs. Taken together, our data show that, depending on patterns of synaptic stimulation, activation of β-ARs can gate the PKA-dependence and persistence of synaptic plasticity. We suggest that this may allow neuromodulatory receptors to fine-tune neural information processing to meet the demands imposed by numerous synaptic activity profiles. This is a form of “metaplasticity” that could control the efficacy of consolidation of hippocampal long-term memories.
The hippocampus importantly contributes to memory function in the mammalian brain (Zola-Morgan et al. 1986; Eichenbaum et al. 1990; Otto and Eichenbaum 1992; Phillips and LeDoux 1992; Remondes and Schuman 2004). It has reciprocal connections with numerous cortical areas, including those responsible for high-level integration of spatial and contextual data from the external environment (Lavenex and Amaral 2000). As such, the hippocampus is well positioned to receive and survey a broad range of information and select behaviorally salient data for long-term storage. Activity-dependent enhancement of hippocampal synaptic strength can store information carried in patterns of afferent neural activity (Bliss and Collingridge 1993; Moser et al. 1998; Nathe and Frank 2003; Whitlock et al. 2006). Substantial evidence suggests that long-term potentiation (LTP) of synaptic strength plays important roles in the formation of long-term memory (LTM) (Doyere and Laroche 1992; Bourtchuladze et al. 1994; Abel and Lattal 2001; Genoux et al. 2002). As such, mechanistic studies of LTP have shed valuable light on how the mammalian brain stores new information.
The hippocampus receives dense noradrenergic projections from the locus coeruleus, a brain structure that can influence many vital brain functions, including attention, sleep, arousal, mood regulation, learning, and memory (Berridge and Waterhouse 2003). Both α- and β-adrenergic receptor subtypes are present on hippocampal neurons (Morrison and Foote 1986; Berridge and Waterhouse 2003), and noradrenaline (NA) acts on hippocampal β-adrenergic receptors (β-ARs) to facilitate the retention and recall of memory (Izquierdo et al. 1998; Ji et al. 2003; Murchison et al. 2004). In humans, stimulation of the noradrenergic neuromodulatory system enhances memory for emotional stimuli, and inhibition of β-ARs prevents this memory enhancement (Cahill et al. 1994; van Stegeren et al. 1998; O’Carroll et al. 1999).
Consistent with the notion that selective enhancement of LTM may occur following β-AR activation, stimulation of β-ARs can also facilitate the persistence of LTP. In areas CA3 and CA1, β-AR activation facilitates the induction of long-lasting LTP when paired with certain patterns of electrical stimulation (Huang and Kandel 1996; Gelinas and Nguyen 2005). However, the mechanisms by which different patterns of stimulation control synaptic responsiveness to β-AR activation are unclear.
β-ARs couple to guanine-nucleotide-binding regulatory Gs proteins to stimulate adenylyl cyclase activity and increase intracellular cAMP (Seeds and Gilman 1971; Maguire et al. 1977). A main target of cAMP signaling is activation of cAMP-dependent protein kinase (PKA), a kinase that is required for some forms of long-lasting LTP and for consolidation of hippocampal LTM (Frey et al. 1993; Abel et al. 1997; Nguyen and Woo 2003). Interestingly, the PKA-dependence of hippocampal LTP displays plasticity: Specific temporal patterns of synaptic stimulation, such as repeated and temporally spaced 100-Hz stimulation, elicit LTP that requires PKA for its expression (Woo et al. 2003). Also, spatial “enrichment” can increase the PKA-dependence of LTP in mice, and this is correlated with improved hippocampal memory function (Duffy et al. 2001). However, it is unclear whether activation of β-ARs can critically gate the PKA-dependence of LTP. In this study, we examine the effects of β-AR activation on LTP generated by various patterns of afferent stimulation in area CA1 of the hippocampus, and we determine the role of PKA in these β-AR-modulated forms of LTP.
Results
Activation of β-ARs in area CA1 of the hippocampus can modulate synaptic responses to electrical stimulation. Because specific patterns of neural activity are thought to encode spatial and contextual information in the mammalian brain, we hypothesized that activating β-ARs could differentially affect the encoding and retention of synaptic information by modulating the expression of LTP elicited by different patterns of electrical stimulation. One important way of modulating the expression of LTP is to control the maintenance of LTP by gating its dependence on key signaling cascades. β-ARs have been importantly linked to stimulation of Gs proteins and subsequent signaling through the cAMP–PKA pathway. To explore the relationship between β-AR activation, PKA-dependence of LTP, and specific patterns of electrical stimulation, we studied “R(AB)” mutant mice. These mice express an inhibitory form of the RIα regulatory subunit of PKA (Abel et al. 1997), and they display a 10-fold reduction of basal hippocampal PKA activity (Young et al. 2006).
β-AR activation rescues LTP elicited by repeated 100-Hz stimulation in hippocampal slices of R(AB) mutant mice and in wild-type slices treated with a PKA inhibitor
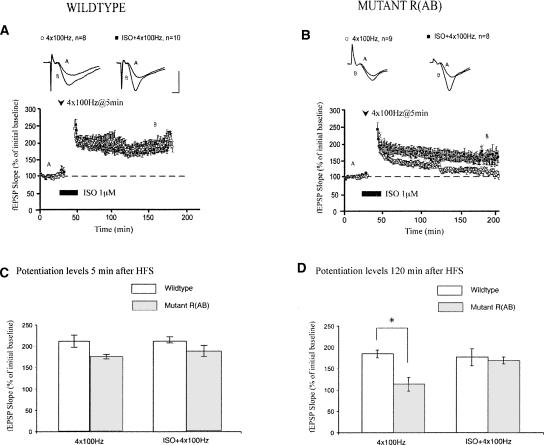
We first investigated the effects of β-AR activation on CA1 LTP induced by application of a strong tetanus protocol, consisting of four trains of 100-Hz high-frequency stimulation (HFS) with a 5-min inter-train interval. This LTP is long lasting and requires PKA (Abel et al. 1997; Woo et al. 2003). We found that application of a β-AR agonist, isoproterenol (ISO), did not alter the induction or the maintenance of this LTP in wild-type slices (Fig. 1A; 5 min after HFS, mean field excitatory postsynaptic potential [fEPSP] slopes were 206 ± 7% in slices treated with ISO and four trains of HFS, P > 0.5 compared with 205 ± 14% in slices treated with four trains of HFS alone; 120 min after HFS, mean fEPSP slopes were 177 ± 11% in slices treated with ISO and four trains of HFS, P > 0.5 compared with 182 ± 17% in slices treated with four trains of HFS alone). Thus, β-AR activation does not affect the expression of LTP induced by a strong tetanus protocol. Furthermore, blockade of β-ARs has not been shown to impair expression of this LTP (Murchison et al. 2004; Schimanski et al. 2007).
Figure 1.
Activation of β-adrenergic receptors rescues impairments of LTP maintenance generated by genetic PKA deficiency. (A) In wild-type mice, application of ISO does not alter LTP generated by four trains of HFS. (B) In mutant mice, maintenance of LTP generated by four trains of LTP is impaired. Application of ISO significantly enhances the maintenance of this LTP. (C) Summary histogram showing that initial potentiation levels are not affected by application of ISO during four trains of HFS in wild-type or mutant mice. (D) Summary histogram showing that the maintenance of LTP is significantly enhanced by ISO application in mutant mice only (*P < 0.05). All sample traces were taken 10 min after commencement of baseline recording and 120 min after HFS. Calibration: 5 mV, 2 msec.
However, when hippocampal PKA activity was genetically reduced, activation of β-ARs potently enhanced the maintenance of this form of LTP. Because R(AB) mice have genetically reduced levels of PKA activity, they display a selective impairment of LTP maintenance following four trains of 100-Hz HFS (Abel et al. 1997; Woo et al. 2002). Two hours after four trains of HFS, mean fEPSP slopes were 116 ± 7% in slices from R(AB) mice, compared with 182 ± 17% in wild-type littermates (P < 0.01) (Fig. 1B). Stimulation of β-ARs with ISO during application of strong tetanus in R(AB) mice rescued this LTP by boosting potentiation to levels seen in slices from wild-type mice (Fig. 1B; mean fEPSP slopes were 175 ± 14% two hours after HFS).
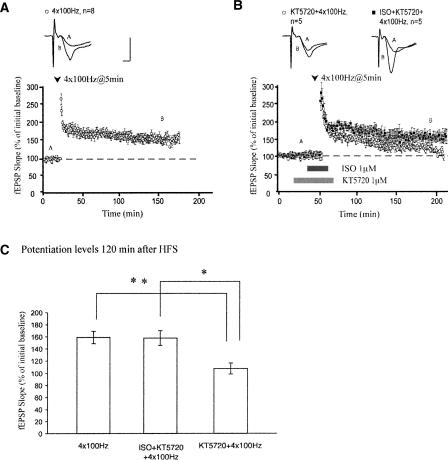
Does β-AR activation also affect LTP in wild-type slices treated with a PKA inhibitor? Treatment with an inhibitor of PKA, KT5720, during four trains of HFS in slices from wild-type C57BL/6 mice impaired maintenance of LTP. Two hours after HFS, mean fEPSP slopes were 107 ± 9% in KT5720-treated slices, compared with 158 ± 10% in slices treated with four trains of HFS alone (P < 0.01; Fig. 2B). Consistent with data obtained from the R(AB) mice, pairing ISO application with four trains of HFS restored LTP maintenance (Fig. 2B; mean fEPSP slopes were 157 ± 12% two hours after HFS, P > 0.5 compared with four trains of HFS alone). ISO application did not affect the amount of potentiation generated by four trains of HFS, but slices treated with KT5720 and ISO displayed enhanced maintenance of LTP compared with slices treated with KT5720 alone. Taken together, these results suggest that, although LTP generated by a strong tetanus protocol is unaffected by activation of β-ARs, stimulation of these receptors can rescue LTP that was disrupted by genetically or pharmacologically induced inhibition of PKA activity.
Figure 2.
Activation of β-adrenergic receptors abolishes impairments of LTP maintenance generated by pharmacologic PKA deficiency. (A) Four trains of HFS generate long-lasting LTP. (B) Application of KT5720 causes LTP elicited by four trains of HFS to decay to levels significantly below KT5720-free controls. Application of ISO enhances the maintenance of this LTP. (C) Summary histogram for these experiments (*P < 0.05, **P < 0.01). All sample traces were taken 10 min after commencement of baseline recording and 120 min after HFS. Calibration: 5 mV, 2 msec.
β-AR activation boosts maintenance of LTP induced by one 100-Hz train in wild-type and R(AB) mutant slices
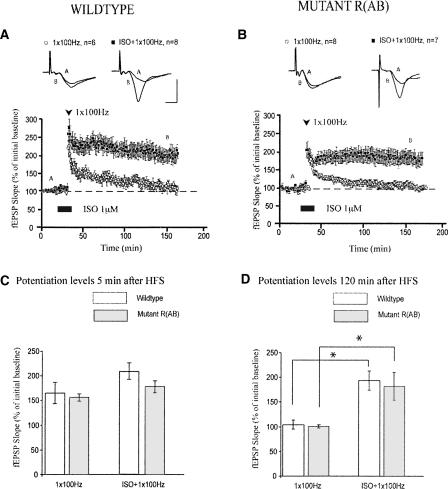
Application of one train of 100-Hz HFS induces a form of LTP that is relatively short lasting and does not require PKA (Huang and Kandel 1994; Duffy et al. 2001). This form of LTP can be converted to long-lasting, protein synthesis-dependent LTP by activation of β-ARs (Gelinas and Nguyen 2005). We next asked whether β-AR activation can enhance this weaker form of LTP during genetic or pharmacologic inhibition of PKA. We found that pairing application of ISO with one train of HFS elicited robust, long-lasting LTP in slices from wild-type mice (Fig. 3A; 120 min after HFS, mean fEPSP slopes were 195 ± 19% in slices treated with ISO and one train of HFS, P < 0.01 compared with 103 ± 10% in slices treated with one train of HFS alone). However, activation of β-ARs during this form of electrical stimulation did not significantly enhance initial levels of potentiation (“short-term potentiation”) (Fig. 3A; 5 min after HFS, mean fEPSP slopes were 214 ± 16% in slices treated with ISO and one train of HFS, P > 0.2 compared with 168 ± 22% in slices treated with one train of HFS alone). Thus, pairing β-AR activation with one train of HFS enhances the maintenance of LTP but not short-term potentiation.
Figure 3.
Slices from R(AB) mice exhibit intact β-adrenergic receptor-dependent enhancement of LTP maintenance. (A) In slices from wild-type mice, application of ISO during one train of HFS induces long-lasting LTP, whereas one train of HFS alone induces decremental LTP. (B) Application of ISO during one train of HFS in mutant slices similarly induces long-lasting LTP. (C) Summary histogram for these experiments comparing levels of potentiation 5 min after HFS. (D) Summary histogram for these experiments comparing levels of potentiation 120 min after HFS (*P < 0.05). All sample traces were taken 10 min after commencement of baseline recording and 120 min after HFS. Calibration: 5 mV, 2 msec.
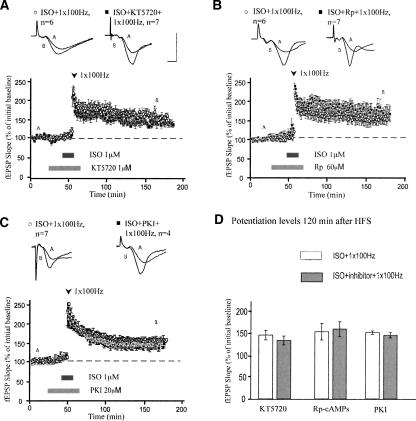
This weak tetanus stimulation protocol also generated stable LTP in the R(AB) mice that was not significantly different from LTP generated in slices from wild-type littermates (Fig. 3C,D). Thus, we further examined the PKA-dependency of this β-AR-enhanced LTP by probing the effects of three different PKA inhibitors: KT5720 (1 μM), Rp-cAMPs (60 μM), and myristoylated membrane-permeant PKI (20 μM). None of these inhibitors significantly affected β-AR-enhanced maintenance of LTP in C57BL/6 mice. Mean fEPSP slopes two hours after HFS were 134 ± 10% for slices treated with KT5720 and ISO, and 146 ± 11% for slices treated with ISO and one train of HFS alone (Fig. 4A; P > 0.4). Slices treated with Rp-cAMPs were potentiated to 160 ± 17% two hours after ISO and HFS, compared with 154 ± 19% in slices treated with ISO and HFS alone (Fig. 4B; P > 0.7). Similarly, PKI had no effect on LTP induced by pairing ISO with one train of HFS (Fig. 4C; mean fEPSP slopes were 146 ± 6% in slices treated with PKI, ISO and one train of HFS compared with 152 ± 4% in slices treated with ISO and one train of HFS alone; P > 0.5). Thus, LTP induced by pairing one train of HFS with β-AR activation does not require PKA for its full expression.
Figure 4.
β-Adrenergic receptor-dependent enhancement of LTP maintenance does not require PKA. (A) Application of KT5720 does not inhibit maintenance of LTP elicited by pairing ISO with one train of HFS. (B) Application of Rp does not inhibit maintenance of LTP elicited by pairing ISO with HFS. (C) Similarly, application of PKI does not inhibit maintenance of LTP elicited by pairing ISO with HFS. (D) Summary histogram for these experiments comparing levels of potentiation 120 min after HFS. All sample traces were taken 10 min after commencement of baseline recording and 120 min after HFS. Calibration: 5 mV, 2 msec.
β-AR activation enables induction of persistent PKA-dependent LTP at a frequency of stimulation that is normally subthreshold for eliciting LTP
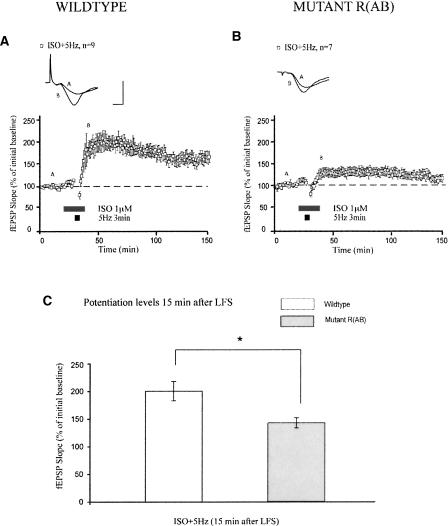
Activation of β-ARs can facilitate LTP induction by patterns of electrical stimulation that are normally unable to potentiate synaptic strength. Application of low-frequency stimulation (LFS) (5 Hz for 3 min) does not persistently alter synaptic strength (Thomas et al. 1996; Woo and Nguyen 2002). However, co-application of ISO with LFS establishes long-lasting LTP (Thomas et al. 1996; Winder et al. 1999; Gelinas and Nguyen 2005). Indeed, we found that pairing ISO with LFS in wild-type slices potentiated mean fEPSP slopes to 201 ± 17% 15 min after LFS (Fig. 5A). This potentiation persisted for at least 120 min after LFS, indicating that activation of β-ARs during LFS also enhances LTP maintenance. However, in mutant R(AB) mice, induction of this form of LTP was blunted (Fig. 5B; mean fEPSP slopes were 143 ± 9% 15 min after LFS, P < 0.05 compared with wild types). Thus, decreased PKA activity impairs, but does not abolish, potentiation generated by pairing β-AR activation with LFS.
Figure 5.
Induction of β-adrenergic receptor-dependent LTP generated by LFS is inhibited by genetic reduction of PKA activity. (A) In slices from wild-type mice, pairing ISO with LFS generates robust LTP. (B) In slices from mutant R(AB) mice, induction of this LTP is significantly decreased. (C) Summary histogram for these experiments comparing levels of potentiation 15 min after LFS (*P < 0.05). All sample traces were taken 10 min after commencement of baseline recording and 15 min after LFS. Calibration: 5 mV, 2 msec.
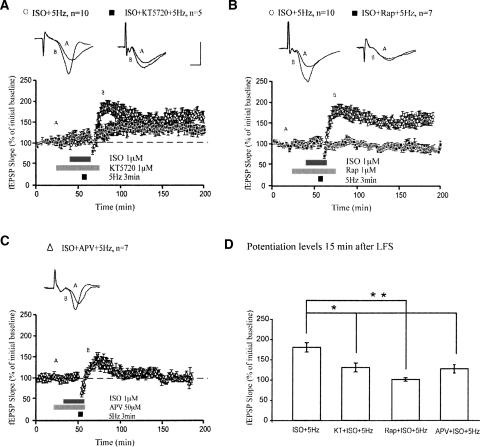
Similarly, pharmacologic inhibition of PKA with KT5720 partially attenuated LTP induced by pairing ISO with LFS in C57BL/6 slices (Fig. 6A; mean fEPSP slopes were 131 ± 13% 15 min after LFS when KT5720 was applied, compared with 180 ± 12% in the absence of KT5720; P < 0.05). These results are consistent with other studies (Thomas et al. 1996; Winder et al. 1999), indicating that this form of LTP is partially dependent on PKA signaling. Thus, we then investigated other signaling cascades that could contribute to this form of β-AR-enhanced LTP. Inhibition of extracellular signal-regulated kinase (ERK) has been shown to partially block LTP generated by pairing ISO application with LFS (Giovannini et al. 2001). We found that pharmacologic inhibition of mammalian target of rapamycin (mTOR) with rapamycin completely blocked induction of this LTP (Fig. 6B; mean fEPSP slopes were 97 ± 6% 15 min after LFS when rapamycin was applied; P < 0.001 compared with ISO + LFS alone). Additionally, NMDA receptor blockade by APV prevented full expression of this form of β-AR-enhanced LTP (Fig. 6C; mean fEPSP slopes were 126 ± 10% 15 min after LFS when APV was applied; P < 0.05 compared with ISO + LFS alone). This dependence on NMDA receptor activation is consistent with previous findings (Thomas et al. 1996). Thus, PKA and mTOR signaling, along with NMDA receptor activation upstream of these signaling pathways, are critical for the expression of LTP elicited by pairing activation of β-ARs with LFS.
Figure 6.
β-Adrenergic receptor-dependent enhancement of LTP induction during LFS requires PKA and mTOR. (A) Application of ISO during LFS induces long-lasting LTP. Co-application of KT5720 significantly blunts induction of this LTP. (B) Co-application of Rap completely blocks induction of this LTP. (C) APV, an NMDA receptor blocker, significantly attenuated expression of this LTP. (D) Summary histogram for these experiments comparing potentiation levels 15 min after LFS (*P < 0.05, **P < 0.01). All sample traces were taken 10 min after commencement of baseline recording and 15 min after LFS. Calibration: 5 mV, 2 msec.
β-AR activation elicits an increase in complex spiking that requires activation of NMDA receptors, but not PKA or mTOR signaling
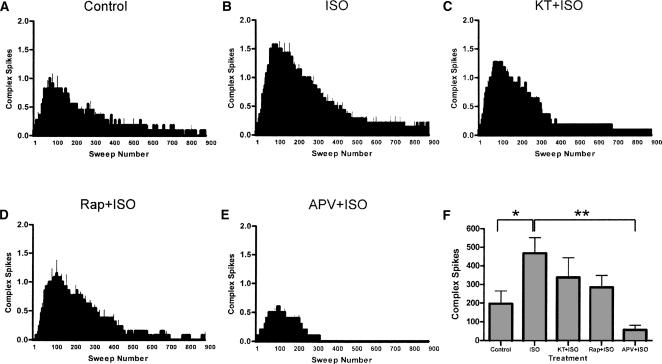
CA1 pyramidal cells produce complex action potential firing patterns during 5-Hz stimulation of Schaeffer collaterals in hippocampal slices, and this complex spiking is significantly increased by β-AR activation (Thomas et al. 1998). These spikes likely activate NMDA receptors by amplifying postsynaptic depolarization; blockade of NMDA receptors effectively reduces LTP induced by 5-Hz LFS during β-AR activation (Thomas et al. 1996). Thus, attenuated complex spiking may be a mechanism for the reduction of this form of LTP seen here following inhibition of PKA and mTOR signaling. It is unknown whether activation of PKA or mTOR signaling is required for complex spiking during 3 min of 5-Hz stimulation in the presence of ISO. To address this question, we measured the total numbers of complex spikes from fEPSP traces obtained during 3 min of 5-Hz LFS. We found that treatment of slices with ISO during this LFS significantly increased the numbers of complex spikes elicited during this stimulation (466 ± 85 in ISO, n = 14 vs. 196 ± 68 in controls, n = 11; P < 0.05; Fig. 7A,B). In slices treated with a PKA inhibitor, KT-5720 (Fig. 7C), no significant change in total numbers of complex spikes was seen (337 ± 105 in KT-5720, n = 11, P > 0.3 compared with the ISO group). Similarly, rapamycin treatment (Fig. 7D) did not significantly alter the numbers of spikes elicited during 3 min of 5-Hz LFS (284 ± 64 in rapamycin, n = 13, P > 0.08 compared with the ISO group). In contrast, APV significantly reduced the numbers of spikes (Fig. 7E; 57 ± 25, n = 9, P < 0.01).
Figure 7.
β-Adrenergic receptor-dependent enhancement of complex spiking requires NMDA receptor activation but not PKA or mTOR signaling. (A) In control slices, 3 min of 5-Hz stimulation (LFS) elicits a modest amount of complex spiking. (B) In the presence of ISO (1 μM), complex spiking was increased significantly. (C) Treatment with KT5720, a PKA inhibitor, did not significantly alter the numbers of complex spikes elicited during LFS in ISO. (D) Rapamycin, an mTOR inhibitor, also did not significantly alter total numbers of spikes. (E) APV significantly reduced the total numbers of complex spikes elicited during LFS. See Results section for mean values of total spike numbers. (F) Summary histogram of total numbers of complex spikes elicited during LFS in these conditions (*P < 0.05, **P < 0.01). Numbers of spikes were counted from individual fEPSP sweeps across the 3-min period of LFS.
These findings indicate that PKA and mTOR signaling are not critical for controlling cell excitability when 3 min of 5-Hz LFS is paired with β-AR activation, leading to enhancement of LTP. Interestingly, a previous study has shown that inhibition of ERK during this stimulation protocol does significantly reduce the number of complex spikes generated (Winder et al. 1999). The results support the notion that the critical sites of action of PKA and mTOR occur downstream of spiking mechanisms, probably at the level of translation (see Gelinas et al. 2007), whereas ERK may be involved in regulation of postsynaptic excitability as well. NMDA receptor activation is necessary for maintaining the increased complex spike firing normally seen during this LFS. Thus, increased complex spiking is not only a consequence of β-AR activation that enables postsynaptic depolarization and NMDA receptor activation critical for enhancing LTP, but this increased complex spiking requires NMDA receptor activation.
Discussion
Our data reveal that synaptic responses to β-AR activation are critically affected by the pattern of electrical stimulation. Patterns of afferent electrical activity determine which temporal phase (induction or maintenance) of LTP is enhanced by β-AR activation, and they also influence which signaling cascades are recruited downstream of the β-AR. We show that transgenic R(AB) mice can be used to elucidate the role of PKA following β-AR activation. Overall, our data suggest that neuromodulatory receptors, such as the β-AR, interact with electrical activity to regulate information processing at the synapse.
β-AR stimulation during a strong, multi-train tetanus protocol did not affect the induction or maintenance of LTP, suggesting that this form of LTP is insensitive to modulation by β-ARs. Because application of repeated 100-Hz HFS strongly stimulates calcium influx and subsequent generation of cAMP via Ca2+-stimulated adenylyl cyclase (Ferguson and Storm 2004), it is possible that downstream signaling mechanisms activated by β-ARs are saturated by this type of electrical stimulation and are unable to further enhance potentiation. On the other hand, application of a weak tetanus protocol would not saturate these mechanisms, allowing for β-AR-dependent enhancement of LTP. Indeed, pairing β-AR activation with a weak tetanus protocol enhances the maintenance of LTP but not short-term potentiation. Taken together, our data demonstrate that activation of β-ARs can contribute to different temporal phases of synaptic plasticity.
If PKA activity is genetically or pharmacologically decreased during strong multi-train HFS, maintenance of this LTP is inhibited. Interestingly, if β-ARs are activated in slices from PKA-deficient R(AB) mice, the maintenance of LTP generated by strong HFS is rescued. Thus, activation of β-ARs can abolish deficiencies in LTP resulting from deficits in PKA signaling, perhaps by recruiting other signaling cascades. There is evidence that β-ARs can recruit extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) signaling cascades (Gelinas and Nguyen 2005; Gelinas et al. 2007), both of which are implicated in the long-term stability of LTP induced by multi-train 100-Hz stimulation (Tang et al. 2002; Kelleher et al. 2004; Sweatt 2004). Although we have not shown that ERK and mTOR are needed for the rescue of multi-train 100-Hz LTP in R(AB) mutants, it is likely that these signaling cascades are involved, because LTP induced by ISO combined with one 100-Hz train elicits significantly increased activation of ERK and mTOR signaling that is well correlated with the magnitude of LTP (Gelinas et al. 2007). Consistent with this, we found that LTP elicited by pairing β-AR activation with one 100-Hz train did not require PKA signaling. Genetic or pharmacologic inhibition of PKA during generation of this LTP did not affect the subsequent induction or maintenance of LTP. Although PKA is a main downstream target of cAMP signaling (Beebe 1994; Nguyen and Woo 2003), PKA-independent activation of ERK and mTOR signaling cascades has been demonstrated. For example, activation of 5-HT7A receptors results in stimulation of cAMP-regulated guanine exchange factors (cAMP-GEFs) (Johnson-Farley et al. 2005). Thus, direct coupling of cAMP to intracellular signaling pathways, such as ERK, via cAMP-GEFs, may occur downstream of the β-AR receptor to enhance the maintenance of LTP.
PKA is critical for LTP induction when a β-AR agonist is paired with 5-Hz LFS. PKA inhibitors significantly blunt this form of LTP. Some residual potentiation is maintained, suggesting that other signaling cascades may contribute to this form of LTP. This residual potentiation was present regardless of whether genetic or pharmacologic methods were used to decrease PKA activity. We found that inhibition of mTOR signaling completely abolished LFS-induced LTP, and previous studies indicate that ERK signaling is also critical (Winder et al. 1999). Taken together, our results suggest that β-ARs recruit PKA during LFS, whereas other signaling cascades may be engaged by stronger forms of synaptic stimulation. Our data also establish that multiple signaling cascades interact, in an activity-sensitive manner, to generate persistent synaptic plasticity that is modulated by β-ARs.
What specific patterns of afferent activity occur in vivo? Field potential oscillations in the range of 3–12 Hz are observed in the hippocampus in vivo (Otto et al. 1991; Stewart et al. 1992). LFS at 5 Hz mimics the endogenous firing patterns of hippocampal neurons. The signaling cascades recruited, and synaptic responses generated, following β-AR activation during 5-Hz LFS in vitro may accurately replicate the subcellular actions of NA on β-ARs in vivo. NA promotes hippocampal electroencephalograhic patterns within the theta frequency range, while suppressing gamma-frequency oscillations (Brown et al. 2005). Furthermore, LTP induced by tetanic stimuli correlates strongly with behavioral learning (Whitlock et al. 2006) and the formation of hippocampal memory (Doyere and Laroche 1992; Bourtchuladze et al. 1994; Abel et al. 1997). Because synaptic responses to β-AR activation are sensitive to the pattern of applied electrical stimulation in vitro, it is likely that the patterning of afferent activity also influences the effects of β-AR activation on LTP and information storage in vivo.
Our results indicate that activation of β-ARs potently modulates LFS-induced forms of synaptic plasticity, enhancing both the induction and maintenance of LTP generated by LFS. Thus, release of NA and subsequent β-AR activation can facilitate the storage of information that may not normally be encoded or retained. Such a mechanism could explain the increased clarity and strength of memories formed during times of intense emotions when NA release is increased (Cahill et al. 1994). Our results have shed new light on how β-AR activation interacts with patterns of neural activity and signal transduction pathways to control the expression of persistent synaptic plasticity that may importantly contribute to information storage. Additionally, because memory enhancement is a key goal of many cognitive rehabilitation programs, our finding that β-AR activation can gate the dependence of synaptic plasticity on PKA, which is a key requirement for making new long-term memories, may provide novel insights on potential molecular drug targets for reducing memory deficits resulting from neurodegenerative diseases.
Materials and Methods
Animals
Female C57BL/6 mice (aged 8–13 wk; Charles River, Montreal, Canada) were used for all experiments unless otherwise indicated. Experiments were also performed on transgenic R(AB) mice (aged 6–10 mo) and age-matched wild-type littermate controls. These mice are maintained in a hemizygous state on a C57BL6/J background and were derived from genetic line 426 characterized for R(AB) transgene expression, synaptic physiology, and behavior (Clegg et al. 1987; Abel et al. 1997; Young et al. 2006). Genotyping was performed by Southern blot using a previously described transgene-specific probe (Abel et al. 1997). All mice were housed under guidelines set forth by the Canadian Council on Animal Care and IACUC.
Electrophysiology
Following cervical dislocation and decapitation of the mice, hippocampi were isolated and transverse slices (400 μM) were cut using a McIlwain slicer (Stoelting). Slices were transferred to an interface recording chamber maintained at 28°C. Oxygenated artificial cerebrospinal fluid (ACSF) containing 125 mM NaCl, 4.4 mM KCl, 1.5 mM MgSO4, 1.0 mM NaH2PO4, 26 mM NaHCO3, 10 mM glucose, 2.5 mM CaCl2 was used for dissection and perfusion. Slices were allowed to recover from slicing for at least an hour before recordings were attempted. Stimulation of Schaeffer collaterals in stratum radiatum elicited fEPSPs, which were recorded with a glass microelectrode positioned in stratum radiatum of area CA1. Baseline test stimuli were applied once per minute at a stimulus intensity set to induce 40% of maximal fEPSP amplitude (0.08-msec pulse width). Electrical stimulation protocols used included LFS (5 Hz for 3 min), and HFS consisting of either one train of 100-Hz (1 sec duration) or four trains of 100 Hz (1 sec duration, 5-min inter-train interval). fEPSPs were monitored with test stimuli for 2 h after induction of LTP. For measurements of numbers of complex spikes elicited during 5-Hz, 3-min stimulation, we followed procedures described in Winder et al. (1999). To count complex spikes on our fEPSP traces during LFS, we excluded the first peak of the fEPSP (Winder et al. 1999); as such, our data likely underestimated the total numbers of spikes.
Drugs
The β-AR agonist, isoproterenol [ISO; R(−)-isoproterenol (+)-bitartrate, 1 μM; Sigma-Aldrich] was prepared daily as a 1 mM stock solution in distilled water. The PKA inhibitors, KT5720 (1 μM; Sigma) and Rp-cAMPs (60 μM; Sigma), were prepared as concentrated stock solutions at 1 mM and 60 mM in DMSO, respectively. Myristoylated PKA inhibitor amide 14–22 (PKI; Calbiochem) was prepared as a 1 mM stock solution in water and used at a bath concentration of 20 μM. 2-Amino-5-phosphonopentanoic acid (D,L-APV; Sigma), an NMDA receptor antagonist, was prepared as a concentrated stock solution in distilled water and diluted to 50 μM in ACSF. After dilution in ACSF, all drugs were bath-applied at a perfusion rate of 1–2 mL/min. The concentrations of PKA inhibitors used here have been shown to effectively block PKA activity in in vitro hippocampal preparations (Huang et al. 2004; Pita-Almenar et al. 2006). Experiments were performed in dimmed light conditions because of drug photosensitivity.
Data analysis
We compared inter-group levels of LTP two hours after LTP induction using initial slope of the fEPSP as an index of synaptic strength (Johnston and Wu 1995). For LTP and complex spiking experiments, two-tailed, unpaired Student’s t-tests were used for statistical comparison between two groups, with Welch correction if standard deviations were significantly different between groups. One-way ANOVA and Tukey-Kramer post-hoc tests were used when comparing three or more groups. When standard deviations differed between multiple groups, the Kruskal-Wallis test with post-hoc Dunn’s multiple comparisons tests were used. The significance criterion was P < 0.05 in all cases. Data are reported as mean ± SEM, with n equal to the number of slices.
Acknowledgments
We thank Clayton Dickson for helpful advice. This research was supported by grants from the Canadian Institutes of Health Research (P.N.), and NIMH and the Human Frontiers Science Program (T.A.), and by M.D.-Ph.D. Studentships (J.G.) from CIHR and the Alberta Heritage Foundation for Medical Research. P.N. is a Faculty Senior Scholar of the Alberta Heritage Foundation and a Distinguished International Scholar of the University of Pennsylvania.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.829208.
References
- Abel T., Lattal K.M. Molecular mechanisms of memory acquisition, consolidation, and retrieval. Curr. Opin. Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Abel T., Nguyen P.V., Barad M., Deuel T.A., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Beebe S.J. The cAMP-dependent protein kinases and cAMP signal transduction. Semin. Cancer Biol. 1994;5:285–294. [PubMed] [Google Scholar]
- Berridge C.W., Waterhouse B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bliss T.V., Collingridge G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R., Frenguelli B., Blendy J., Cioffi D., Schutz G., Silva A.J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brown R.A., Walling S.G., Milway J.S., Harley C.W. Locus ceruleus activation suppresses feedforward interneurons and reduces beta-gamma electroencephalogram frequencies while it enhances theta frequencies in rat dentate gyrus. J. Neurosci. 2005;25:1985–1991. doi: 10.1523/JNEUROSCI.4307-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L., Prins B., Weber M., McGaugh J.L. β-Adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Clegg C.H., Correll L.A., Cadd G.G., McKnight G.S. Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J. Biol. Chem. 1987;262:13111–13119. [PubMed] [Google Scholar]
- Doyere V., Laroche S. Linear relationship between the maintenance of hippocampal long-term potentiation and retention of an associative memory. Hippocampus. 1992;2:39–48. doi: 10.1002/hipo.450020106. [DOI] [PubMed] [Google Scholar]
- Duffy S.N., Craddock K.J., Abel T., Nguyen P.V. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn. Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H., Stewart C., Morris R.G. Hippocampal representation in place learning. J. Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson G.D., Storm D.R. Why calcium-stimulated adenylyl cyclases? Physiology. 2004;19:271–276. doi: 10.1152/physiol.00010.2004. [DOI] [PubMed] [Google Scholar]
- Frey U., Huang Y.Y., Kandel E.R. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Gelinas J.N., Nguyen P.V. β-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J. Neurosci. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas J.N., Banko J.L., Hou L., Sonenberg N., Weeber E.J., Klann E., Nguyen P.V. ERK and mTOR signaling couple β-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J. Biol. Chem. 2007;282:27527–27535. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- Genoux D., Haditsch U., Knobloch M., Michalon A., Storm D., Mansuy I.M. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Giovannini M.G., Blitzer R.D., Wong T., Asoma K., Tsokas P., Morrison J.H., Iyengar R., Landau E.M. Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation. J. Neurosci. 2001;21:7053–7062. doi: 10.1523/JNEUROSCI.21-18-07053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.Y., Kandel E.R. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn. Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Huang Y.Y., Kandel E.R. Modulation of both the early and the late phase of mossy fiber LTP by the activation of β-adrenergic receptors. Neuron. 1996;16:611–617. doi: 10.1016/s0896-6273(00)80080-x. [DOI] [PubMed] [Google Scholar]
- Huang Y.Y., Pittenger C., Kandel E.R. A form of long-lasting, learning-related synaptic plasticity in the hippocampus induced by heterosynaptic low-frequency pairing. Proc. Natl. Acad. Sci. 2004;101:859–864. doi: 10.1073/pnas.2237201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I., Medina J.H., Izquierdo L.A., Barros D.M., de Souza M.M., Mello e Souza T. Short- and long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiol. Learn. Mem. 1998;69:219–224. doi: 10.1006/nlme.1998.3825. [DOI] [PubMed] [Google Scholar]
- Ji J.Z., Wang X.M., Li B.M. Deficit in long-term contextual fear memory induced by blockade of β-adrenoceptors in hippocampal CA1 region. Eur. J. Neurosci. 2003;17:1947–1952. doi: 10.1046/j.1460-9568.2003.02620.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Farley N.N., Kertesy S.B., Dubyak G.R., Cowen D.S. Enhanced activation of Akt and extracellular-regulated kinase pathways by simultaneous occupancy of Gq-coupled 5-HT2A receptors and Gs-coupled 5-HT7A receptors in PC12 cells. J. Neurochem. 2005;92:72–82. doi: 10.1111/j.1471-4159.2004.02832.x. [DOI] [PubMed] [Google Scholar]
- Johnston D., Wu S. Foundations of cellular neurophysiology. MIT Press; Cambridge, MA: 1995. Extracellular field recordings; pp. 423–435. [Google Scholar]
- Kelleher R.J., Govindarajan A., Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lavenex P., Amaral D.G. Hippocampal-neocortical interaction: A hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Maguire M.E., Ross E.M., Gilman A.G. β-Adrenergic receptor: Ligand binding properties and the interaction with adenylyl cyclase. Adv. Cyclic Nucleotide Res. 1977;8:1–83. [PubMed] [Google Scholar]
- Morrison J.H., Foote S.L. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J. Comp. Neurol. 1986;243:117–138. doi: 10.1002/cne.902430110. [DOI] [PubMed] [Google Scholar]
- Moser E.I., Krobert K.A., Moser M.B., Morris R.G. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Murchison C.F., Zhang X.Y., Zhang W.P., Ouyang M., Lee A., Thomas S.A. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nathe A.R., Frank L.M. Making space for rats: From synapse to place code. Neuron. 2003;39:730–731. doi: 10.1016/s0896-6273(03)00537-3. [DOI] [PubMed] [Google Scholar]
- Nguyen P.V., Woo N.H. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog. Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- O’Carroll R.E., Drysdale E., Cahill L., Shajahan P., Ebmeier K.P. Memory for emotional material: A comparison of central versus peripheral beta blockade. J. Psychopharmacol. 1999;13:32–39. doi: 10.1177/026988119901300104. [DOI] [PubMed] [Google Scholar]
- Otto T., Eichenbaum H. Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: Evidence for hippocampal processing in recognition memory. Hippocampus. 1992;2:323–334. doi: 10.1002/hipo.450020310. [DOI] [PubMed] [Google Scholar]
- Otto T., Eichenbaum H., Wiener S.I., Wible C.G. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pita-Almenar J.D., Sol Collado M., Colbert C.M., Eskin A. Different mechanisms exist for the plasticity of glutamate reuptake during early long-term potentiation (LTP) and late LTP. J. Neurosci. 2006;26:10461–10471. doi: 10.1523/JNEUROSCI.2579-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondes M., Schuman E.M. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Schimanski L.A., Ali D.W., Baker G.B., Nguyen P.V. Impaired hippocampal LTP in inbred mouse strains can be rescued by β-adrenergic receptor activation. Eur. J. Neurosci. 2007;25:1589–1598. doi: 10.1111/j.1460-9568.2007.05376.x. [DOI] [PubMed] [Google Scholar]
- Seeds N., Gilman A. Norepinephrine stimulated increase of cyclic AMP levels in developing mouse brain cell cultures. Science. 1971;174:292. doi: 10.1126/science.174.4006.292. [DOI] [PubMed] [Google Scholar]
- Stewart M., Quirk G.J., Barry M., Fox S.E. Firing relations of medial entorhinal neurons to the hippocampal theta rhythm in urethane anesthetized and walking rats. Exp. Brain Res. 1992;90:21–28. doi: 10.1007/BF00229252. [DOI] [PubMed] [Google Scholar]
- Sweatt J.D. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tang S.J., Reis G., Kang H., Gingras A.C., Sonenberg N., Schuman E.M. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.J., Moody T.D., Makhinson M., O’Dell T.J. Activity-dependent β-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 1996;17:475–482. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- Thomas M.J., Watabe A.M., Moody T.D., Makhinson M., O’Dell T.J. Postsynaptic complex spike bursting enables the induction of LTP by theta frequency synaptic stimulation. J. Neurosci. 1998;18:7118–7126. doi: 10.1523/JNEUROSCI.18-18-07118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren A.H., Everaerd W., Cahill L., McGaugh J.L., Gooren L.J. Memory for emotional events: Differential effects of centrally versus peripherally acting beta-blocking agents. Psychopharmacology. 1998;138:305–310. doi: 10.1007/s002130050675. [DOI] [PubMed] [Google Scholar]
- Whitlock J.R., Heynen A.J., Shuler M.G., Bear M.F. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Winder D.G., Martin K.C., Muzzio I.A., Rohrer D., Chruscinski A., Kobilka B., Kandel E.R. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by β-adrenergic receptors. Neuron. 1999;24:715–726. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- Woo N.H., Nguyen P.V. “Silent” metaplasticity of the late phase of long-term potentiation requires protein phosphatases. Learn. Mem. 2002;9:202–213. doi: 10.1101/lm.498402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo N.H., Abel T., Nguyen P.V. Genetic and pharmacological demonstration of a role for cyclic AMP-dependent protein kinase-mediated suppression of protein phosphatases in gating the expression of late LTP. Eur. J. Neurosci. 2002;16:1871–1876. doi: 10.1046/j.1460-9568.2002.02260.x. [DOI] [PubMed] [Google Scholar]
- Woo N.H., Duffy S.N., Abel T., Nguyen P.V. Temporal spacing of synaptic stimulation critically modulates the dependence of LTP on cyclic AMP-dependent protein kinase. Hippocampus. 2003;13:293–300. doi: 10.1002/hipo.10086. [DOI] [PubMed] [Google Scholar]
- Young J.Z., Isiegas C., Abel T., Nguyen P.V. Metaplasticity of the late-phase of long-term potentiation: A critical role for protein kinase A in synaptic tagging. Eur. J. Neurosci. 2006;23:1784–1794. doi: 10.1111/j.1460-9568.2006.04707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L.R., Amaral D.G. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J. Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]